
Assertion:It is safe to stir 1M \[AgN{{O}_{3}}\] solution with a copper spoon.
Reason: \[\begin{align}
& E_{A{{g}^{+}}/Ag}^{-}=0.80V \\
& E_{C{{u}^{2+}}/Cu}^{-}=0.34V \\
\end{align}\]
A.Both Assertion and Reason are true and Reason is the correct explanation of Assertion.
B.Both Assertion and Reason are true but Reason is not the correct explanation of Assertion
C.Assertion is true but Reason is false
D.Assertion is false but Reason is true
E.Both assertion and reason are false
Answer
590.4k+ views
Hint: The element with high electrode potential value undergoes reduction and electrode with low electrode potential undergoes oxidation. These two electrodes combine and form a complete cell. If the emf of the cell is positive then the cell exists.
Complete answer:
We cannot stir a silver nitrate solution with a copper spoon because Copper is more reactive (less electrode potential) than silver (high electrode potential). Copper will displace silver from silver nitrate and forms blue color copper nitrate solution.
Therefore the Assertion is false.
Coming to reason.
\[\begin{align}
& E_{A{{g}^{+}}/Ag}^{-}=0.80V \\
& E_{C{{u}^{2+}}/Cu}^{-}=0.34V \\
\end{align}\]
The cell representation for the silver and copper is as follows according to the given values.
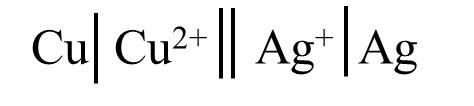
\[\begin{align}
& E_{cell}^{-}=E_{A{{g}^{+}}/Ag}^{-}-E_{C{{u}^{2+}}/Cu}^{-} \\
& \text{ }=\text{ }0.80-0.34 \\
& \text{ = 0}\text{.46} \\
\end{align}\]
\[E_{cell}^{-}\] is positive so the cell exists. So, Reason is true.
Therefore it is not safe to stir the silver nitrate solution with a copper spoon.
\[Cu+AgN{{O}_{3}}\to CuN{{O}_{3}}+Ag\]
In the question the Assertion is false and reason is true.
So, the correct option is D.
Note:
As time goes on a white precipitate of Silver metal (Ag) will be formed and the Solution changes it color gradually to blue in color owing to the formation of Copper Nitrate (\[CuN{{O}_{3}}\]). Copper acts as anode and silver acts as cathode. Copper loses its electrons and silver gains electrons.
Complete answer:
We cannot stir a silver nitrate solution with a copper spoon because Copper is more reactive (less electrode potential) than silver (high electrode potential). Copper will displace silver from silver nitrate and forms blue color copper nitrate solution.
Therefore the Assertion is false.
Coming to reason.
\[\begin{align}
& E_{A{{g}^{+}}/Ag}^{-}=0.80V \\
& E_{C{{u}^{2+}}/Cu}^{-}=0.34V \\
\end{align}\]
The cell representation for the silver and copper is as follows according to the given values.
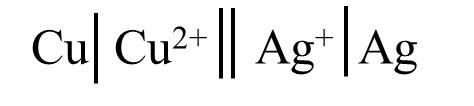
\[\begin{align}
& E_{cell}^{-}=E_{A{{g}^{+}}/Ag}^{-}-E_{C{{u}^{2+}}/Cu}^{-} \\
& \text{ }=\text{ }0.80-0.34 \\
& \text{ = 0}\text{.46} \\
\end{align}\]
\[E_{cell}^{-}\] is positive so the cell exists. So, Reason is true.
Therefore it is not safe to stir the silver nitrate solution with a copper spoon.
\[Cu+AgN{{O}_{3}}\to CuN{{O}_{3}}+Ag\]
In the question the Assertion is false and reason is true.
So, the correct option is D.
Note:
As time goes on a white precipitate of Silver metal (Ag) will be formed and the Solution changes it color gradually to blue in color owing to the formation of Copper Nitrate (\[CuN{{O}_{3}}\]). Copper acts as anode and silver acts as cathode. Copper loses its electrons and silver gains electrons.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




