
Assertion: Transparent soaps are made by dissolving soaps in ethanol.
Reason: Ethanol makes things invisible
A. Both Assertion and Reason are correct and Reason is the correct explanation for Assertion.
B. Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion
C. Assertion is correct but Reason is incorrect
D. Assertion is incorrect but reason is correct
Answer
569.1k+ views
Hint: We can say that the process by which triglycerides are reacted with sodium or potassium hydroxide to form glycerol and a fatty acid salt called soap. The triglycerides are mostly animal fats or vegetable oils. A hard soap can be produced with sodium hydroxide and a soft soap can be produced with potassium hydroxide.
Complete step by step answer:
We must remember that the soaps are the salts of long-chain carboxylic acids called as fatty acids salts. The length of the carbon chain of the fatty salts decides the solubility of soap.
The synthesis of soap is given below,
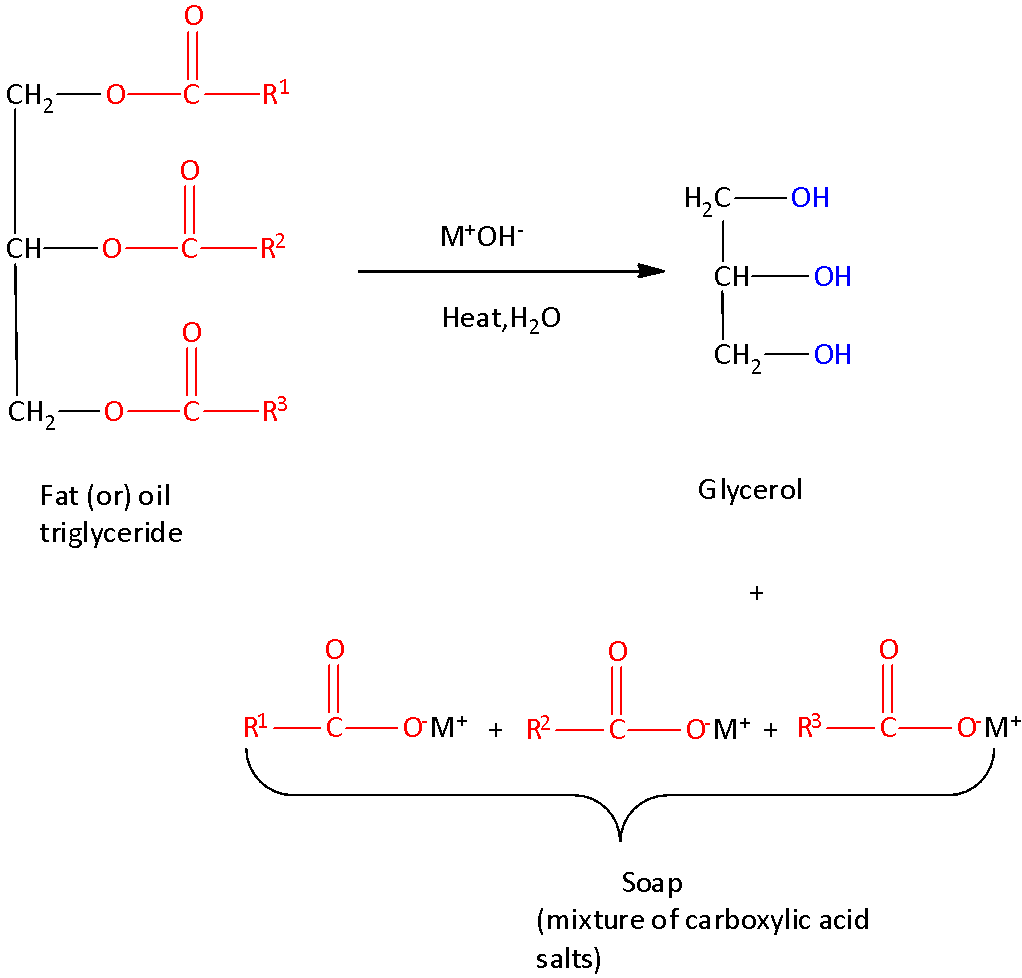
The long hydrocarbon chain or tail is similar to an alkane and is soluble in nonpolar compounds due to the presence of London dispersion forces. However, it is repelled by water and is hydrophobic in nature.
The carboxylate end of the molecule is polar and has the ability to bond hydrogen to water and this region of soap is hydrophilic.
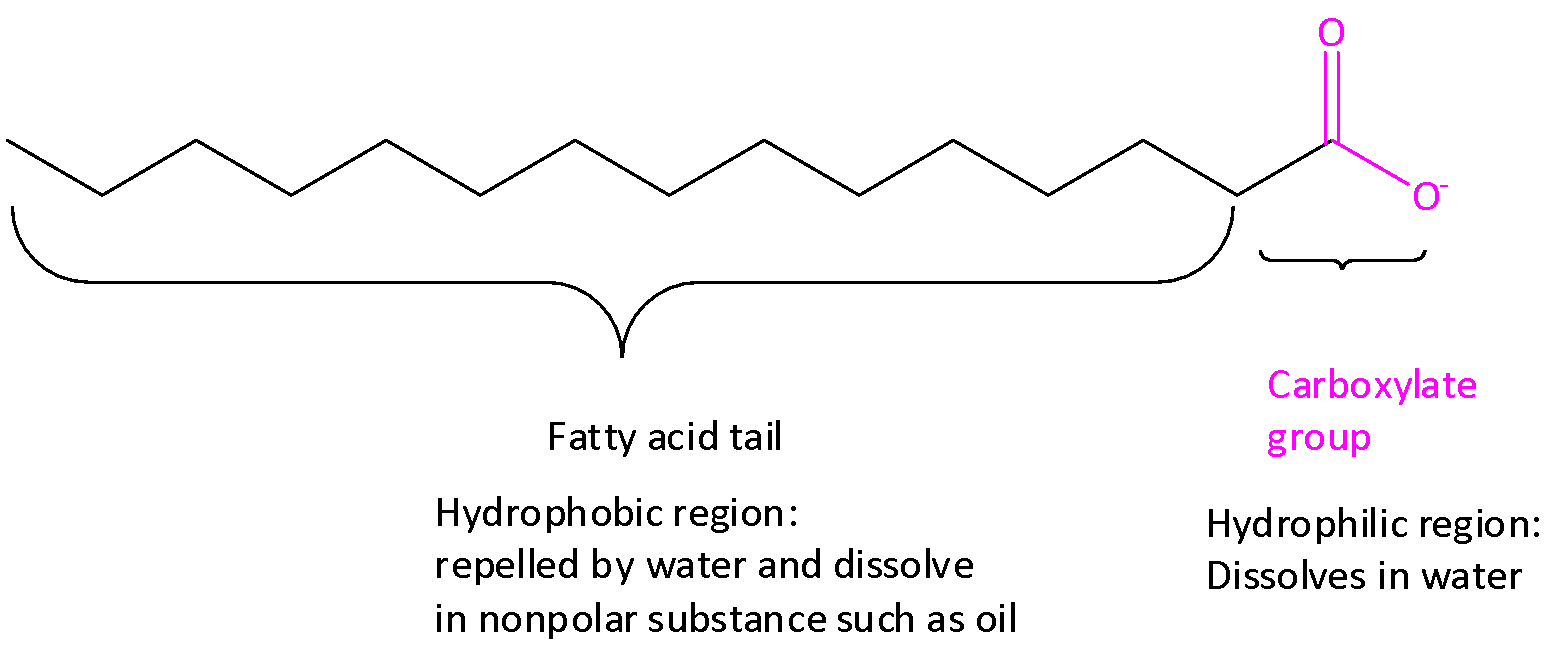 When soap is dissolved in water, the carboxylate end dissolves, but the water repels the long fatty acid tails. When grease or oil is present in the mixture, the fatty acid tails dissolve in these nonpolar substances. The formation of tiny spheres in which the carboxylate ends of the soap forms the outside of the sphere and interacts with water.
When soap is dissolved in water, the carboxylate end dissolves, but the water repels the long fatty acid tails. When grease or oil is present in the mixture, the fatty acid tails dissolve in these nonpolar substances. The formation of tiny spheres in which the carboxylate ends of the soap forms the outside of the sphere and interacts with water.
The fatty acid tails, along with the dissolved grease or oil, are surrounded within the center of the spheres. These spheres are called micelles.
From the explanation, we understood that the water-soluble salts of fatty acids are soaps. They are synthesized from oils and fats (or) by their fatty acids by reacting them with strong alkali (or) base. We can make transparent soaps by dissolving them in the solvent ethanol and the extra amount of solvent is evaporated. We have to remember that we make use of ethanol to form transparent soaps but ethanol does not make things invisible. During the preparation of soap, the alcohol makes the soap to get dissolved into clear and amber liquid. So Assertion is correct but reason is incorrect.
So, the correct answer is Option D.
Note: We have to remember that transparent soap contains a high amount of glycerin. It is often known as glycerin soap. An example of transparent soap is Pears. Generally, glycerin attracts moisture and so these soaps are more moisturizing when compared to soaps that are opaque, which has less glycerin content.
Complete step by step answer:
We must remember that the soaps are the salts of long-chain carboxylic acids called as fatty acids salts. The length of the carbon chain of the fatty salts decides the solubility of soap.
The synthesis of soap is given below,

The long hydrocarbon chain or tail is similar to an alkane and is soluble in nonpolar compounds due to the presence of London dispersion forces. However, it is repelled by water and is hydrophobic in nature.
The carboxylate end of the molecule is polar and has the ability to bond hydrogen to water and this region of soap is hydrophilic.

The fatty acid tails, along with the dissolved grease or oil, are surrounded within the center of the spheres. These spheres are called micelles.
From the explanation, we understood that the water-soluble salts of fatty acids are soaps. They are synthesized from oils and fats (or) by their fatty acids by reacting them with strong alkali (or) base. We can make transparent soaps by dissolving them in the solvent ethanol and the extra amount of solvent is evaporated. We have to remember that we make use of ethanol to form transparent soaps but ethanol does not make things invisible. During the preparation of soap, the alcohol makes the soap to get dissolved into clear and amber liquid. So Assertion is correct but reason is incorrect.
So, the correct answer is Option D.
Note: We have to remember that transparent soap contains a high amount of glycerin. It is often known as glycerin soap. An example of transparent soap is Pears. Generally, glycerin attracts moisture and so these soaps are more moisturizing when compared to soaps that are opaque, which has less glycerin content.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




