
Assertion: Sodium potassium salt of tartaric acid is known as Rochelle's salt. The IUPAC name of Rochelle's salt is sodium-potassium-2,3-dihydroxy butane-1,4-dioate.
Reason: Rochelle’s salt is used as a complexing agent in Tollens reagent.
(a)- Both assertion and reason are correct and reason is the correct explanation for the assertion.
(b)- Both assertion and reason are correct and the reason is not the correct explanation for the assertion.
(c)- Assertion is correct but the reason is incorrect
(d)- Both Assertion and reason are incorrect.
Answer
558.9k+ views
Hint: The other name of Rochelle’s salt is potassium sodium tartrate. It is a component of the test in which we can differentiate between the aromatic aldehyde and aliphatic aldehyde, in this test, there are two types of solution used.
Complete step by step answer:
Rochelle's salt in which there are two ions sodium and potassium, and it is a double salt of tartaric acid. The formula of tartaric acid is ${{C}_{4}}{{H}_{6}}{{O}_{6}}$ and its structure is given below:
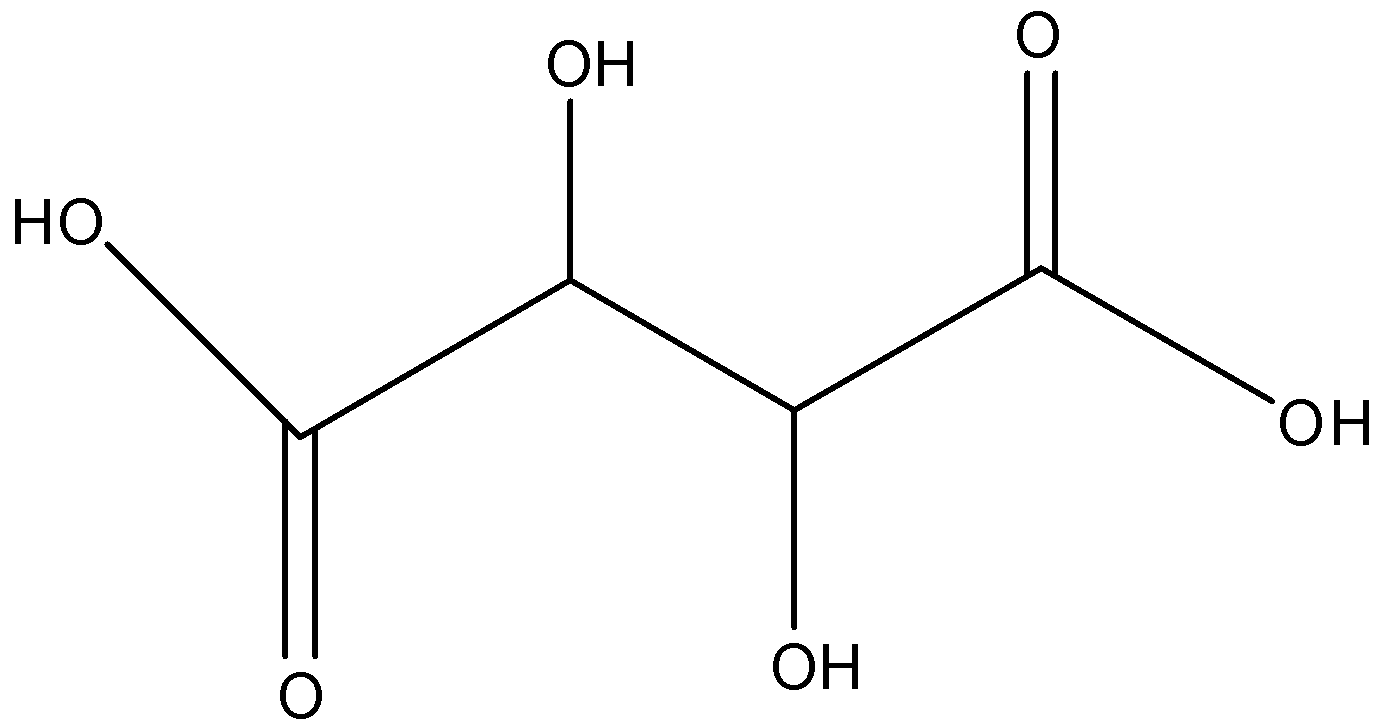
In Rochelle’s salt, both the terminal hydrogen atoms are replaced with sodium and potassium ions, so the formula of Rochelle’s salt is $KNa{{C}_{4}}{{H}_{4}}{{O}_{6}}$ and its structure is given below:
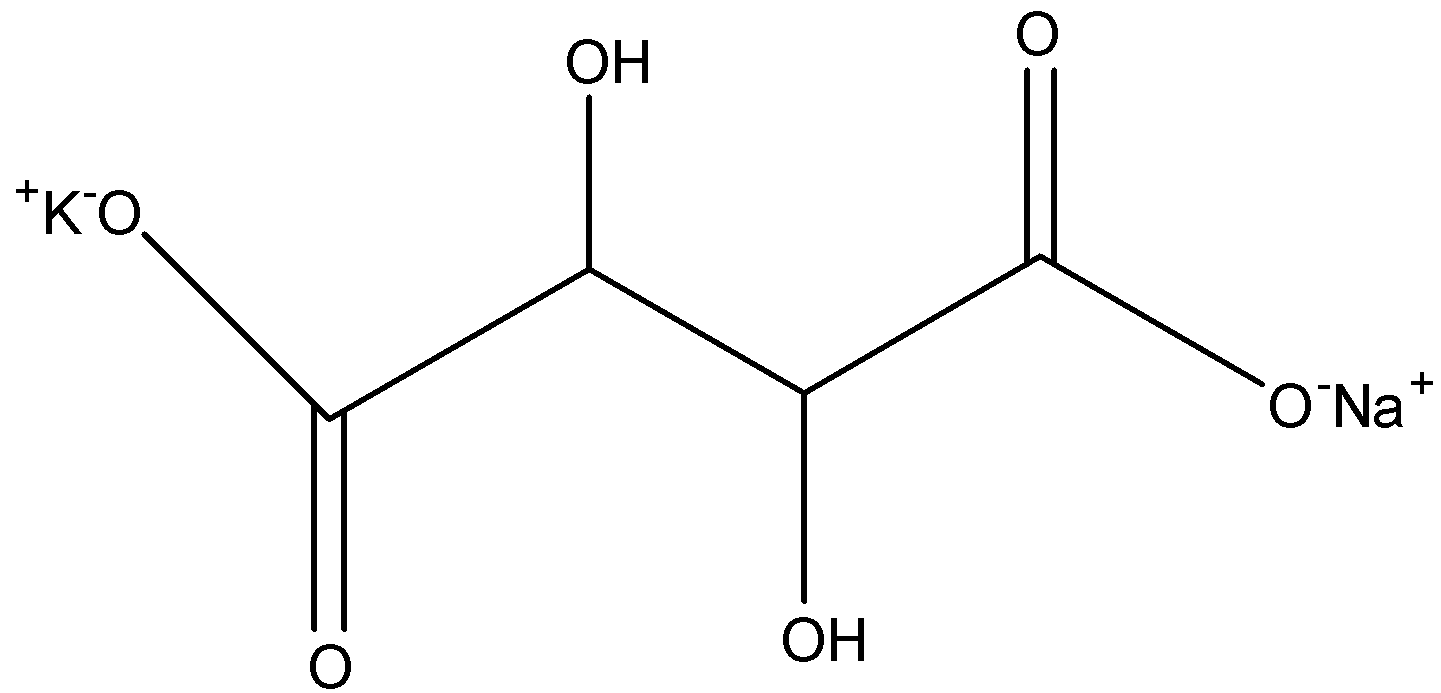
Therefore, the IUPAC name is sodium-potassium-2,3-dihydroxy butane-1,4-dioate.
It is a component of the test in which we can differentiate between the aromatic aldehyde and aliphatic aldehyde. This test is known as Fehling's solution which is a solution of $CuS{{O}_{4}}$ containing some Rochelle's salt. There are two solutions named solution A which is the solution of Rochelle's salt and solution B which is the solution of $CuS{{O}_{4}}$. Only aliphatic aldehydes give this test and aromatic aldehydes do not give this test. When aliphatic aldehyde reacts there is a formation of a red precipitate.
So, the assertion is correct but the reason is not correct. The correct option is option “C” .
Note: Tollens test is also a test used to differentiate between the aldehydes and the ketones. This test is known as the silver mirror test and the compositions of this test are silver nitrate and ammoniacal solution.
Complete step by step answer:
Rochelle's salt in which there are two ions sodium and potassium, and it is a double salt of tartaric acid. The formula of tartaric acid is ${{C}_{4}}{{H}_{6}}{{O}_{6}}$ and its structure is given below:

In Rochelle’s salt, both the terminal hydrogen atoms are replaced with sodium and potassium ions, so the formula of Rochelle’s salt is $KNa{{C}_{4}}{{H}_{4}}{{O}_{6}}$ and its structure is given below:

Therefore, the IUPAC name is sodium-potassium-2,3-dihydroxy butane-1,4-dioate.
It is a component of the test in which we can differentiate between the aromatic aldehyde and aliphatic aldehyde. This test is known as Fehling's solution which is a solution of $CuS{{O}_{4}}$ containing some Rochelle's salt. There are two solutions named solution A which is the solution of Rochelle's salt and solution B which is the solution of $CuS{{O}_{4}}$. Only aliphatic aldehydes give this test and aromatic aldehydes do not give this test. When aliphatic aldehyde reacts there is a formation of a red precipitate.
So, the assertion is correct but the reason is not correct. The correct option is option “C” .
Note: Tollens test is also a test used to differentiate between the aldehydes and the ketones. This test is known as the silver mirror test and the compositions of this test are silver nitrate and ammoniacal solution.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




