
Assertion: Phenol forms 2,4,6-tribromophenol on treatment with $B{r_2}$ water at $373K$.
Reason: Phenol is o,p-directing group.
A.Both Assertion and Reason are correct and Reason is the correct explanation for Assertion.
B.Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion.
C.Assertion is correct but Reason is incorrect.
D.Both Assertion and Reason are incorrect.
Answer
571.2k+ views
Hint: Phenol when reacted with different compounds shows different reactions. Even reactions with the same compound under different conditions yield different reactions. Phenol when reacted with a group.
Complete Step-by-step Solution:
Let us see what will happen when phenol is treated with bromine water at $373K$.
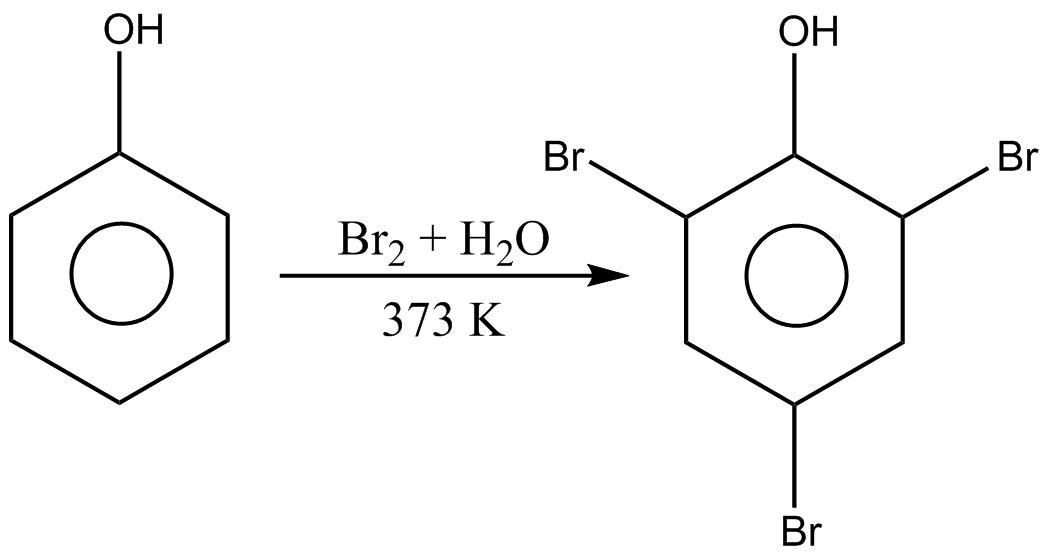
We can see that when phenol is reacted with $B{r_2}$ water in conditions as mentioned we get 2,4,6-tribromophenol as the final product.
We know that even with the same reactant under different conditions, the reaction would have given different products. So if these reactions were to take place in presence of $C{S_2}$ ( carbon disulphide) or $CC{l_4}$ ( carbon tetrachloride).
But when aqueous solution is taken, the phenoxide ion formed is highly reactive and capable of directing the substituent at all the three( two ortho and one para) positions. Hence the substituent group gets attached at the positions available. And we get 2,4,6-tribromophenol as our final product.
Assertion is correct.
The reason is that phenol is ortho-para directing. This is because of the presence of the $ - OH$ group, which donates electron making phenol partially negative at ortho-and para positions. This opens up the possibility of the electrophile attack. However, this reason is not the correct or sufficient explanation of the above Assertion.
Hence, the correct option is B.
Note: We have to keep in mind under what conditions the reaction is taking place. And that it doesn’t depend solely on the reactant but also on the surrounding conditions.
Complete Step-by-step Solution:
Let us see what will happen when phenol is treated with bromine water at $373K$.

We can see that when phenol is reacted with $B{r_2}$ water in conditions as mentioned we get 2,4,6-tribromophenol as the final product.
We know that even with the same reactant under different conditions, the reaction would have given different products. So if these reactions were to take place in presence of $C{S_2}$ ( carbon disulphide) or $CC{l_4}$ ( carbon tetrachloride).
But when aqueous solution is taken, the phenoxide ion formed is highly reactive and capable of directing the substituent at all the three( two ortho and one para) positions. Hence the substituent group gets attached at the positions available. And we get 2,4,6-tribromophenol as our final product.
Assertion is correct.
The reason is that phenol is ortho-para directing. This is because of the presence of the $ - OH$ group, which donates electron making phenol partially negative at ortho-and para positions. This opens up the possibility of the electrophile attack. However, this reason is not the correct or sufficient explanation of the above Assertion.
Hence, the correct option is B.
Note: We have to keep in mind under what conditions the reaction is taking place. And that it doesn’t depend solely on the reactant but also on the surrounding conditions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




