
Assertion
In the pressure-temperature (P-T) phase diagram of water, the slope of the melting curve is found to be negative.
Reason
Ice contracts on melting to water.
A. Both Assertion and Reason are correct and Reason is the correct explanation for Assertion.
B Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion.
C. Assertion is correct but Reason is incorrect.
D. Both Assertion and Reason are incorrect.
Answer
574.2k+ views
Hint:In this type of questions, we will first check if the assertion is correct. Then, we will check if the reason is correct. Then, we will find if the reason can be taken as a correct explanation for the assertion.
Complete answer:
The pressure-temperature (P-T) phase diagram of water is given below.
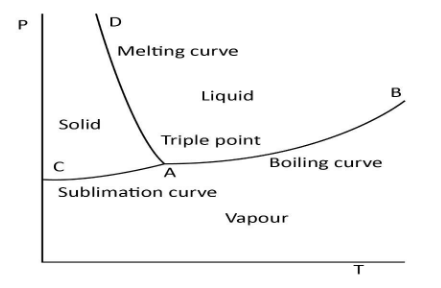
As we know, the supply of heat to ice results in the melting of ice to water. By supplying heat, we increase the temperature of ice. From the melting curve, it is clear that the pressure decreases with an increase in temperature during melting. Thus, the slope of the melting curve is negative. Hence, our assertion is proved to be true.
It is to be noted that the density of ice is lesser than water. We can easily prove it by taking a bottle full of water. We will freeze the bottle with the cap on it. If we take the bottle after one day of freezing, we will see a broken bottle. The bottle broke because the volume of ice exceeded the maximum volume the bottle could occupy. Hence, we conclude that when the same amount of water gets converted to ice, the volume increases. A high volume implies low density. Hence, ice has a lesser density than water. So, the distance separating the molecules of ice is greater than the distance separating the molecules of water. Therefore, as the ice melts into water, it contracts. So, the reason is true.
Since ice contracts on melting, it is clear to us that an increase in temperature will result in a reduction in the pressure during the melting process. Hence, the reason gives a proper explanation for the assertion.
Since, both assertion and reason are correct and reason is the correct explanation for assertion, option (A) is correct.
Note:Since ice is a solid substance and water is a liquid, we might think that ice expands on melting, which is wrong. While the majority of solids expand on heating, ice, cast iron and rubber contract on heating.
Complete answer:
The pressure-temperature (P-T) phase diagram of water is given below.

As we know, the supply of heat to ice results in the melting of ice to water. By supplying heat, we increase the temperature of ice. From the melting curve, it is clear that the pressure decreases with an increase in temperature during melting. Thus, the slope of the melting curve is negative. Hence, our assertion is proved to be true.
It is to be noted that the density of ice is lesser than water. We can easily prove it by taking a bottle full of water. We will freeze the bottle with the cap on it. If we take the bottle after one day of freezing, we will see a broken bottle. The bottle broke because the volume of ice exceeded the maximum volume the bottle could occupy. Hence, we conclude that when the same amount of water gets converted to ice, the volume increases. A high volume implies low density. Hence, ice has a lesser density than water. So, the distance separating the molecules of ice is greater than the distance separating the molecules of water. Therefore, as the ice melts into water, it contracts. So, the reason is true.
Since ice contracts on melting, it is clear to us that an increase in temperature will result in a reduction in the pressure during the melting process. Hence, the reason gives a proper explanation for the assertion.
Since, both assertion and reason are correct and reason is the correct explanation for assertion, option (A) is correct.
Note:Since ice is a solid substance and water is a liquid, we might think that ice expands on melting, which is wrong. While the majority of solids expand on heating, ice, cast iron and rubber contract on heating.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




