
Assertion: Empirical formula of glucose is $\text{HCHO}$.
Reason: Molecular formula of glucose will also be equal to $\text{HCHO}$.
A. Both Assertion and Reason are correct but Reason is the correct explanation for Assertion
B. Both Assertion and Reason are correct but Reason is not the correct explanation for Assertion
C. Assertion is correct but Reason is incorrect
D. Both Assertion and Reason are incorrect
Answer
526.1k+ views
Hint: Write the difference between the two terms molecular and empirical formula to check glucose. Write the general formula of carbohydrates as glucose is carbohydrate. It will be distinguished after knowing that. Check also the molar mass of both its empirical and molecular formula.
Complete answer:
Let us discuss the difference between molecular formula and empirical formula is:
Let us discuss about glucose and its empirical formula and molecular formula,
Glucose is a carbohydrate as it contains carbon, hydrogen and oxygen. Carbohydrates have general formula as ${{\text{C}}_{\text{x}}}{{\left( {{\text{H}}_{2}}\text{O} \right)}_{\text{y}}}$. It is a monosaccharide, an aldose, a hexose and a reducing agent too. Glucose is synthesized by chlorophyll in plants using sunlight from the sun and carbon dioxide $\left( \text{C}{{\text{O}}_{2}} \right)$ from the air. The reaction is $\text{6C}{{\text{O}}_{2}}+12{{\text{H}}_{2}}\text{O}\xrightarrow[\text{chlorophyll}]{\text{light}}{{\text{C}}_{6}}{{\text{H}}_{12}}{{\text{O}}_{6}}+6{{\text{O}}_{2}}+\text{6}{{\text{H}}_{2}}\text{O}$. The structure of glucose is
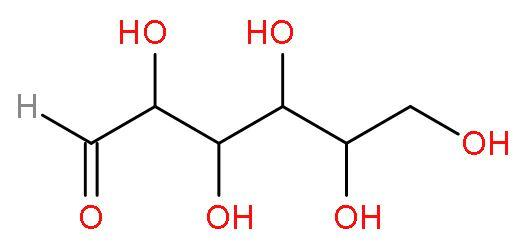
It contains six carbon atoms, twelfth hydrogen atoms and six oxygen atoms. The chemical formula is ${{\text{C}}_{6}}{{\text{H}}_{12}}{{\text{O}}_{6}}$. It can also be represented as ${{\left( \text{HCHO} \right)}_{6}}$. The exact molar mass of glucose is 180 grams. The molecular formula of glucose is different with the empirical formula of glucose.
The correct answer is option ‘c’, Assertion is correct but Reason is incorrect.
Note:
All the compounds which fit in the formula ${{\text{C}}_{\text{x}}}{{\left( {{\text{H}}_{2}}\text{O} \right)}_{\text{y}}}$, are not carbohydrates like acetic acid or $\text{C}{{\text{H}}_{3}}\text{COOH}$, it can be written as ${{\text{C}}_{2}}{{\left( {{\text{H}}_{2}}\text{O} \right)}_{2}}$ but is not a carbohydrate. Similarly, rhamnose is a carbohydrate with chemical formula ${{\text{C}}_{6}}{{\text{H}}_{12}}{{\text{O}}_{5}}$ does not fit this definition of ${{\text{C}}_{\text{x}}}{{\left( {{\text{H}}_{2}}\text{O} \right)}_{\text{y}}}$.
Complete answer:
Let us discuss the difference between molecular formula and empirical formula is:
| EMPIRICAL FORMULA | MOLECULAR FORMULA |
| It is the simplest form of formula of a compound. | It is the actual and real formula of a compound. |
| Does not give exact molecular mass. | It gives exact molecular mass. |
| Gives the simplest ratio of atoms in a molecule. | Gives the exact number of atoms of element present in the compound. |
| Can predict the type of atoms present and repeating units in polymer. | Can predict the oxidation state of atoms of reactants and products. |
| For simpler molecules, polymers are its uses. | Gives name to covalent compounds only. |
Let us discuss about glucose and its empirical formula and molecular formula,
Glucose is a carbohydrate as it contains carbon, hydrogen and oxygen. Carbohydrates have general formula as ${{\text{C}}_{\text{x}}}{{\left( {{\text{H}}_{2}}\text{O} \right)}_{\text{y}}}$. It is a monosaccharide, an aldose, a hexose and a reducing agent too. Glucose is synthesized by chlorophyll in plants using sunlight from the sun and carbon dioxide $\left( \text{C}{{\text{O}}_{2}} \right)$ from the air. The reaction is $\text{6C}{{\text{O}}_{2}}+12{{\text{H}}_{2}}\text{O}\xrightarrow[\text{chlorophyll}]{\text{light}}{{\text{C}}_{6}}{{\text{H}}_{12}}{{\text{O}}_{6}}+6{{\text{O}}_{2}}+\text{6}{{\text{H}}_{2}}\text{O}$. The structure of glucose is

It contains six carbon atoms, twelfth hydrogen atoms and six oxygen atoms. The chemical formula is ${{\text{C}}_{6}}{{\text{H}}_{12}}{{\text{O}}_{6}}$. It can also be represented as ${{\left( \text{HCHO} \right)}_{6}}$. The exact molar mass of glucose is 180 grams. The molecular formula of glucose is different with the empirical formula of glucose.
The correct answer is option ‘c’, Assertion is correct but Reason is incorrect.
Note:
All the compounds which fit in the formula ${{\text{C}}_{\text{x}}}{{\left( {{\text{H}}_{2}}\text{O} \right)}_{\text{y}}}$, are not carbohydrates like acetic acid or $\text{C}{{\text{H}}_{3}}\text{COOH}$, it can be written as ${{\text{C}}_{2}}{{\left( {{\text{H}}_{2}}\text{O} \right)}_{2}}$ but is not a carbohydrate. Similarly, rhamnose is a carbohydrate with chemical formula ${{\text{C}}_{6}}{{\text{H}}_{12}}{{\text{O}}_{5}}$ does not fit this definition of ${{\text{C}}_{\text{x}}}{{\left( {{\text{H}}_{2}}\text{O} \right)}_{\text{y}}}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




