
Assertion: Addition of one equivalent of HCl to 1,3-butene at 804${}^{o}C$ gives 3- chloro- 1 – butene as a major product.
Reason: 3- chloro- 1- butene is a kinetically controlled product.
A. Both Assertion and Reason are correct and Reason is the correct explanation of Assertion.
B. Both Assertion and Reason are correct but Reason is not the correct explanation of Assertion.
C. Assertion is correct but Reason is incorrect.
D. Both Assertion and Reason are incorrect.
Answer
578.4k+ views
Hint: As per markovnikov’s rule at lower temperature kinetically controlled products are going to form as the major products. At higher temperature the reaction is going to favor the formation of thermodynamically controlled products.
Complete answer:
- In Assertion it is given that Addition of one equivalent of HCl to 1,3-butene at 80 ${}^{o}C$ gives 3- chloro- 1 – butane as a major product. The chemical reaction is as follows.
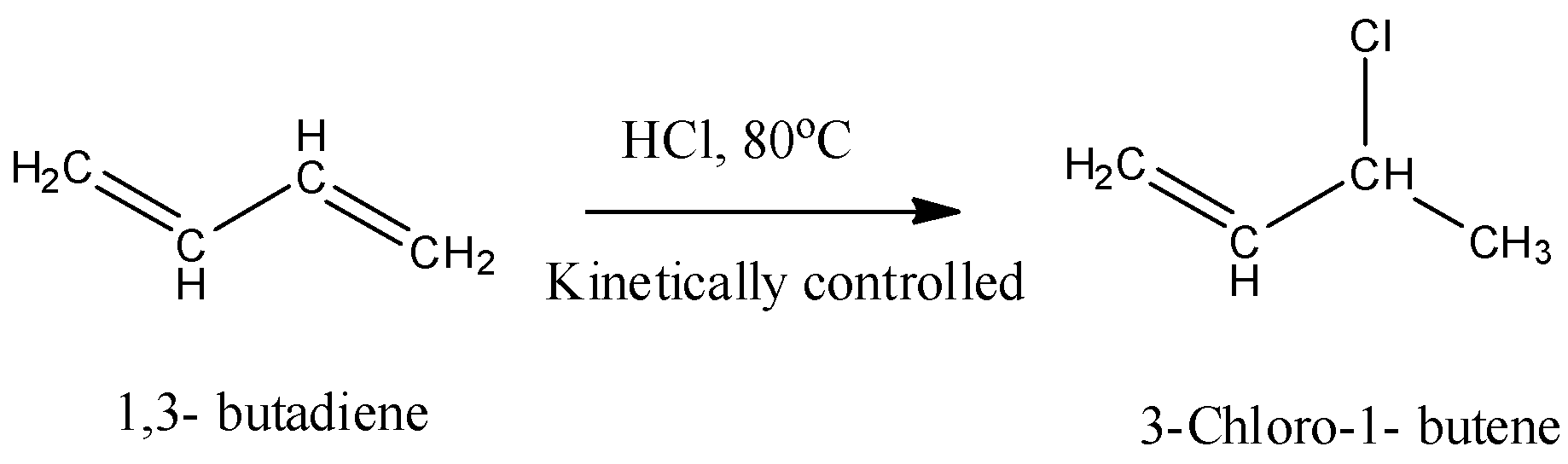
- Yes it is true at lower temperature 1,3-butadiene undergoes follows markovnikov’s rule and produces kinetically controlled product as the major product.
- At higher temperature (100 ${}^{o}C$ or above) the reaction favors the formation of thermodynamically controlled products as the major product and the chemical reaction is as follows.
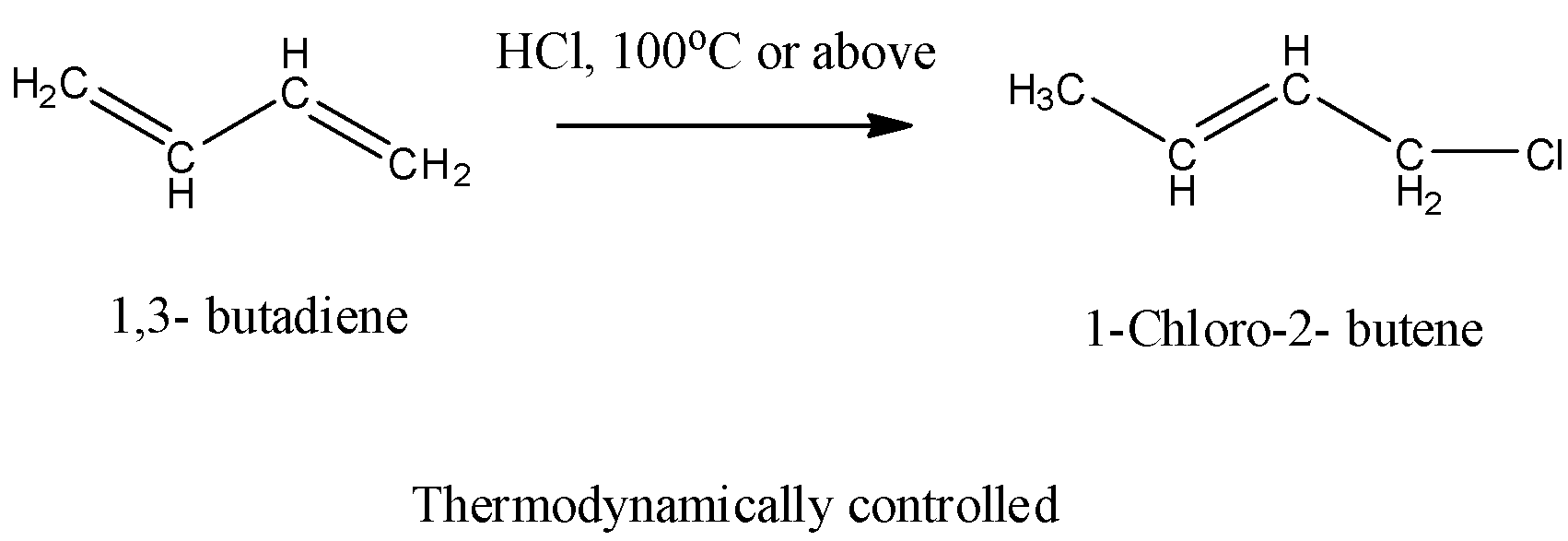
- Therefore Assertion is the correct statement.
- Coming to reason 3- chloro- 1- butane is a kinetically controlled product. Yes it suits the assertion.
- Therefore both Assertion and Reason are correct and Reason is the correct explanation of Assertion
So, the correct option is A.
Note:
In thermodynamically controlled reaction temperature is going to play a big role and produces a product which is different from kinetically controlled reaction.
The product formed in the reaction of 1,3-butadiene with HCl under kinetically controlled condition is 3- chloro- 1 – butene.
The product formed in the reaction of 1,3-butadiene with HCl under thermodynamically controlled condition is 1- chloro- 3 – butene.
Complete answer:
- In Assertion it is given that Addition of one equivalent of HCl to 1,3-butene at 80 ${}^{o}C$ gives 3- chloro- 1 – butane as a major product. The chemical reaction is as follows.

- Yes it is true at lower temperature 1,3-butadiene undergoes follows markovnikov’s rule and produces kinetically controlled product as the major product.
- At higher temperature (100 ${}^{o}C$ or above) the reaction favors the formation of thermodynamically controlled products as the major product and the chemical reaction is as follows.

- Therefore Assertion is the correct statement.
- Coming to reason 3- chloro- 1- butane is a kinetically controlled product. Yes it suits the assertion.
- Therefore both Assertion and Reason are correct and Reason is the correct explanation of Assertion
So, the correct option is A.
Note:
In thermodynamically controlled reaction temperature is going to play a big role and produces a product which is different from kinetically controlled reaction.
The product formed in the reaction of 1,3-butadiene with HCl under kinetically controlled condition is 3- chloro- 1 – butene.
The product formed in the reaction of 1,3-butadiene with HCl under thermodynamically controlled condition is 1- chloro- 3 – butene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




