
Assertion (A): Friedel-Crafts reaction of benzene with n-propyl chloride gives isopropylbenzene.
Reason (R): Benzene undergoes electrophilic substitution readily.
A.Both Assertion and Reason are true and Reason is the correct explanation of Assertion.
B.Both Assertion and Reason are true and Reason is not the correct explanation of Assertion.
C.Assertion is true but Reason is False.
D.Both Assertion and Reason are False.
Answer
578.1k+ views
Hint:In the electrophilic substitution reaction, there is presence of a functional group like $H$ atom on the ring or compound, it will get replaced by an electrophile. $Cl,Br$ act like an electrophile. It is a type of three step mechanism reaction. In case of Friedel-crafts reaction there is addition of an electrophile to the compound.
Complete step by step answer:
-Now, we will discuss both the statements in detail,
-In the first, we have to discuss the Friedel-crafts reaction of benzene with n-propyl chloride. Friedel-crafts reaction is of two types that are Alkylation and acylation reactions
-Let us have a look at the reaction of Friedel-crafts reaction of benzene. This reaction is an example of Friedel-crafts alkylation reaction.
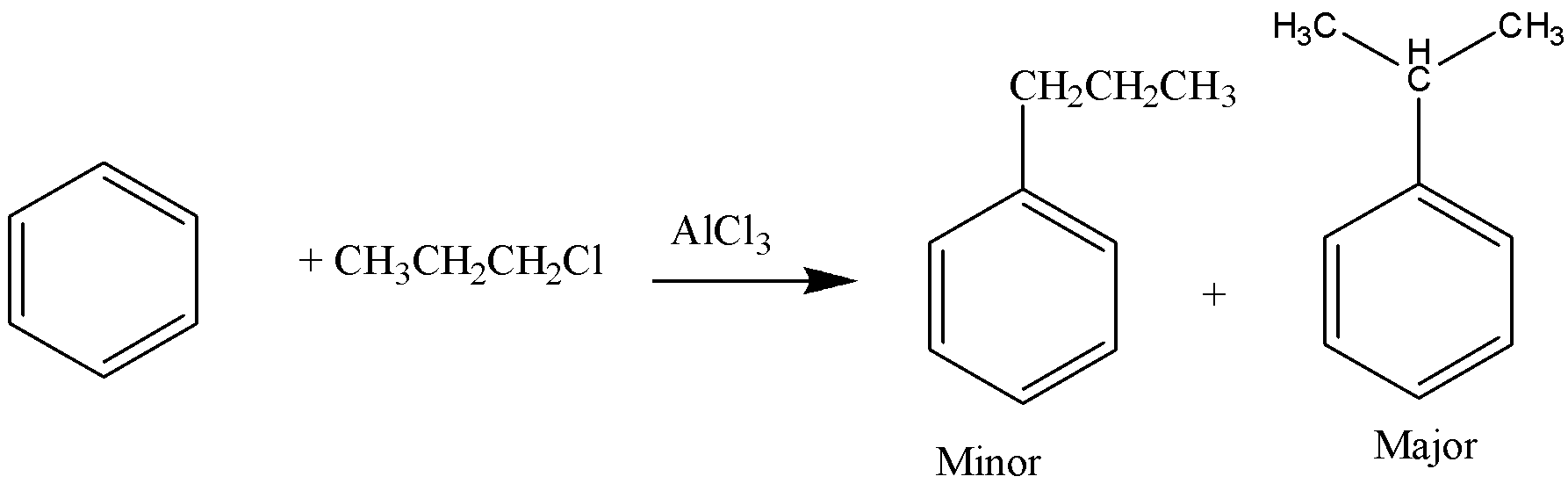
-In this reaction, $AlC{l_3}$ acts as a Lewis acid catalyst as it will increase the speed of reaction. The reactants are benzene and n-propyl chloride. When the benzene reacts with n-propyl chloride in the presence of aluminium chloride a catalyst, a carbonium ion is formed and it leads to the formation of two products as shown in the figure. The major product formed is known as isopropyl benzene as a propyl group is present in the form of branching.
-Here, we can say that the given assertion is the correct statement.
-In the second, we have to discuss the electrophilic substitution of benzene. Let us take an example of a benzene ring having a hydrogen atom. The reaction is shown as follows:
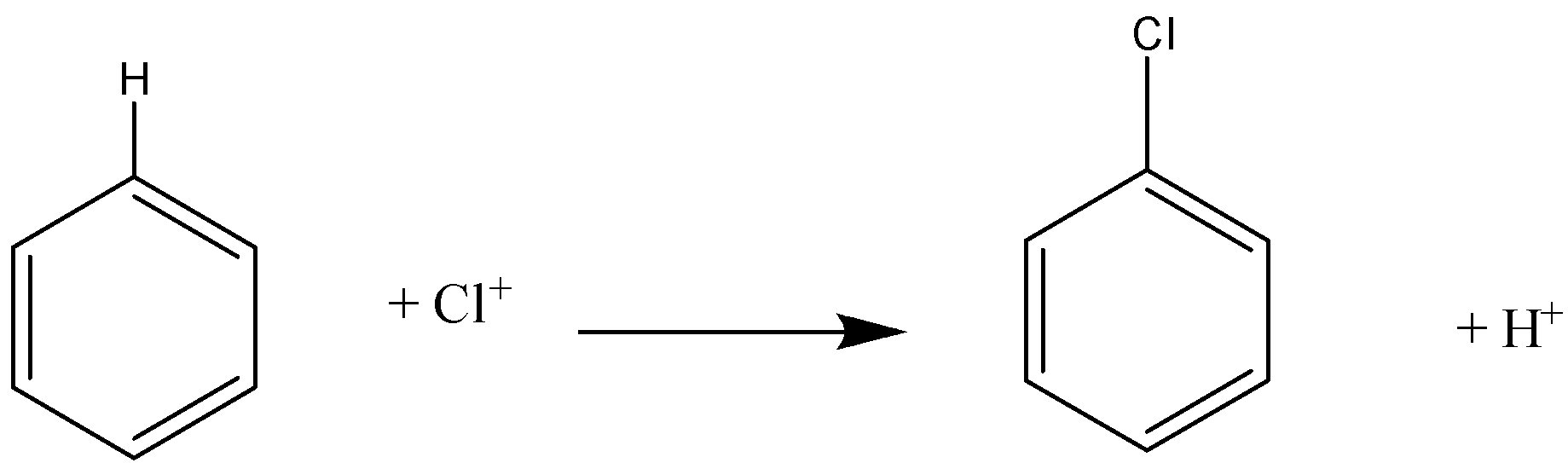
-In this reaction, $C{l^ + }$ acts as an electrophile, and on the reaction it will replace the hydrogen atom, and a chlorobenzene product will be formed.
-Now, we can say that the statement given in the reason is also correct.
-So, in the end we can conclude that the assertion and reason are correct but reason is not the correct explanation of assertion.
The correct answer is option B.
Note:
We have come across a term catalyst. Catalyst is a kind of substance which is used in the reaction to increase the rate without any consumption in the chemical reactions. The rate is increased by reducing the activation energy or by the change in reaction mechanism.
Complete step by step answer:
-Now, we will discuss both the statements in detail,
-In the first, we have to discuss the Friedel-crafts reaction of benzene with n-propyl chloride. Friedel-crafts reaction is of two types that are Alkylation and acylation reactions
-Let us have a look at the reaction of Friedel-crafts reaction of benzene. This reaction is an example of Friedel-crafts alkylation reaction.

-In this reaction, $AlC{l_3}$ acts as a Lewis acid catalyst as it will increase the speed of reaction. The reactants are benzene and n-propyl chloride. When the benzene reacts with n-propyl chloride in the presence of aluminium chloride a catalyst, a carbonium ion is formed and it leads to the formation of two products as shown in the figure. The major product formed is known as isopropyl benzene as a propyl group is present in the form of branching.
-Here, we can say that the given assertion is the correct statement.
-In the second, we have to discuss the electrophilic substitution of benzene. Let us take an example of a benzene ring having a hydrogen atom. The reaction is shown as follows:

-In this reaction, $C{l^ + }$ acts as an electrophile, and on the reaction it will replace the hydrogen atom, and a chlorobenzene product will be formed.
-Now, we can say that the statement given in the reason is also correct.
-So, in the end we can conclude that the assertion and reason are correct but reason is not the correct explanation of assertion.
The correct answer is option B.
Note:
We have come across a term catalyst. Catalyst is a kind of substance which is used in the reaction to increase the rate without any consumption in the chemical reactions. The rate is increased by reducing the activation energy or by the change in reaction mechanism.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




