
Assertion: $2,2 - $Dimethylbutane does not have any tertiary carbon atoms.
Reason: Tertiary carbon atoms are attached to three carbon atoms.
A.) Both Assertion and Reason are correct and Reason is the correct explanation for the Assertion.
B.) Both Assertion and Reason are correct but Reason is not the correct explanation for the Assertion.
C.) Assertion is correct but reason is incorrect.
D.) Both Assertion and reason are incorrect.
Answer
589.5k+ views
Hint: To solve this question we first need to know that tertiary carbons are those carbons which are attached to three other carbons. Also in simple alkanes, the tertiary carbon will have only one hydrogen attached to it.
Complete step by step answer:
First we will know the type of carbons that are there. These are primary, secondary, tertiary and quaternary.
Here, primary carbons are those carbons which are directly attached to only one carbon group.
Secondary carbons are those carbons which are directly attached to only two carbons.
Tertiary carbons are those carbons which are directly attached to three other carbon groups.
Quaternary carbons are those carbons which are directly attached to four other carbons groups.
Here, in this question first we will draw the structure of $2,2 - $Dimethylbutane. It can be draw as follows:
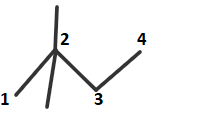
In this structure, as we can see carbon$ - 1$ is directly attached to just one carbon (that is carbon-$2$) so it is a primary carbon.
In case of carbon$ - 2$ as we can see it is directly attached to four other carbons. So, carbon$ - 2$ is a
quaternary carbon.
In case of carbon $ - 3$, as we can see that it is directly attached to two other carbons. So, it is
secondary carbon.
In case of carbon $ - 4$, as it is directly attached to only one carbon so it is primary carbon.
For Assertion:
As we can see here that no carbon is tertiary in nature that is no carbon is there which is
attached to three other carbons. Hence, Assertion is correct.
For Reason:
As we know that tertiary carbon is a carbon which is attached to other three carbons. Hence,
Reason is correct and it is the right explanation of Assertion.
Hence, Option A.) is the correct option.
Note:
These types of questions can be solved by other tricks also. That is, if there is a simple compound with no functional group then we can say that the primary carbon will have $3$ carbons with it, secondary carbon will have $3$ hydrogen with it, tertiary carbon will have $1$ hydrogen with it and quaternary carbon will have no hydrogen attached with it.
Complete step by step answer:
First we will know the type of carbons that are there. These are primary, secondary, tertiary and quaternary.
Here, primary carbons are those carbons which are directly attached to only one carbon group.
Secondary carbons are those carbons which are directly attached to only two carbons.
Tertiary carbons are those carbons which are directly attached to three other carbon groups.
Quaternary carbons are those carbons which are directly attached to four other carbons groups.
Here, in this question first we will draw the structure of $2,2 - $Dimethylbutane. It can be draw as follows:

In this structure, as we can see carbon$ - 1$ is directly attached to just one carbon (that is carbon-$2$) so it is a primary carbon.
In case of carbon$ - 2$ as we can see it is directly attached to four other carbons. So, carbon$ - 2$ is a
quaternary carbon.
In case of carbon $ - 3$, as we can see that it is directly attached to two other carbons. So, it is
secondary carbon.
In case of carbon $ - 4$, as it is directly attached to only one carbon so it is primary carbon.
For Assertion:
As we can see here that no carbon is tertiary in nature that is no carbon is there which is
attached to three other carbons. Hence, Assertion is correct.
For Reason:
As we know that tertiary carbon is a carbon which is attached to other three carbons. Hence,
Reason is correct and it is the right explanation of Assertion.
Hence, Option A.) is the correct option.
Note:
These types of questions can be solved by other tricks also. That is, if there is a simple compound with no functional group then we can say that the primary carbon will have $3$ carbons with it, secondary carbon will have $3$ hydrogen with it, tertiary carbon will have $1$ hydrogen with it and quaternary carbon will have no hydrogen attached with it.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




