
Aspirin is obtained by the reaction of acetyl chloride with
(A) Ethanol
(B) Phenol
(C) Salicylic acid
(D) Benzoic acid
Answer
581.7k+ views
Hint: We know that aspirin is also known as acetylsalicylic acid . Aspirin is a very important compound which is used to make many medicines. The molecular formula of aspirin is ${C_9}{H_8}{O_4}$. Aspirin is used as an analgesic .
Complete step by step answer:
As we know that the aspirin is formed by a special type of reaction known as esterification reaction. Acetyl chloride is a very reactive compound. Acetyl chloride reacts with Salicylic acid vigorously in the presence of pyridine to form acetylsalicylic acid or the aspirin . Pyridine is used in the reaction because the nitrogen in the pyridine is a nucleophilic catalyst. Aspirin can also be formed by the reaction of salicylic acid and acetic anhydride. So from the above explanation it is clear that aspirin is formed by the reaction of acetyl chloride and salicylic acid.
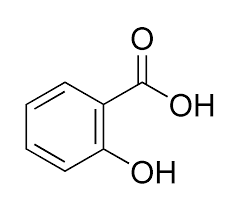
Fig:Salicylic acid
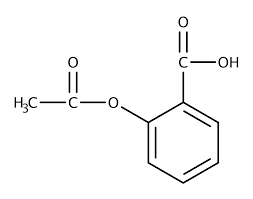
Fig: Aspirin or acetylsalicylic acid
So the correct answer of the question is : (C) Salicylic acid
Additional information:
Aspirin or acetylsalicylic acid is used to cure pain, fever and inflammation. Aspirin is a non narcotic drug. It is used as a painkiller. Aspirin is also used to cure rheumatoid arthritis, pericarditis, and Kawasaki disease. Some examples of narcotic drugs are codeine , morphine and heroin. Non narcotic drugs are offered as non prescription drugs.
Note:
Always remember that aspirin is formed by the esterification reaction. Acetyl chloride reacts with salicylic acid in the presence of pyridine to form aspirin. Aspirin is also known as acetylsalicylic acid. It is a non narcotic drug which is used to cure many diseases .
Complete step by step answer:
As we know that the aspirin is formed by a special type of reaction known as esterification reaction. Acetyl chloride is a very reactive compound. Acetyl chloride reacts with Salicylic acid vigorously in the presence of pyridine to form acetylsalicylic acid or the aspirin . Pyridine is used in the reaction because the nitrogen in the pyridine is a nucleophilic catalyst. Aspirin can also be formed by the reaction of salicylic acid and acetic anhydride. So from the above explanation it is clear that aspirin is formed by the reaction of acetyl chloride and salicylic acid.

Fig:Salicylic acid

Fig: Aspirin or acetylsalicylic acid
So the correct answer of the question is : (C) Salicylic acid
Additional information:
Aspirin or acetylsalicylic acid is used to cure pain, fever and inflammation. Aspirin is a non narcotic drug. It is used as a painkiller. Aspirin is also used to cure rheumatoid arthritis, pericarditis, and Kawasaki disease. Some examples of narcotic drugs are codeine , morphine and heroin. Non narcotic drugs are offered as non prescription drugs.
Note:
Always remember that aspirin is formed by the esterification reaction. Acetyl chloride reacts with salicylic acid in the presence of pyridine to form aspirin. Aspirin is also known as acetylsalicylic acid. It is a non narcotic drug which is used to cure many diseases .
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




