
Aspartame, an artificial sweetener is a peptide and has the following structure. Which of the following is correct about the molecule?
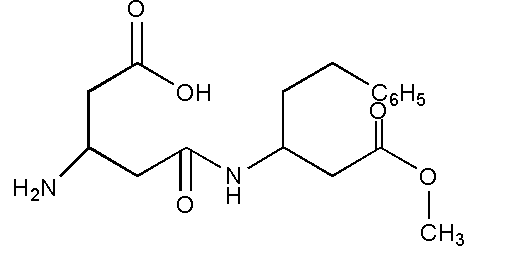
This question has multiple correct options.
A. It has four functional groups.
B. It has three functional groups
C. On hydrolysis it produces only one amino acid.
D. On hydrolysis it produces a mixture of amino acids.

Answer
565.2k+ views
Hint: Aspartame is used as a substitute of sugar as an artificial sweetener in foods and beverages. The aspartame contains two amino acids which are joined together by a peptide bond.
Complete step by step answer:
Aspartame is an artificial sweetener which is a non-saccharides which is 200 times sweeter than the sucrose. It is commonly used as a sugar substitute added in food and beverages. Aspartame is a methyl ester of aspartic acid. Aspartame is a formed in 1965 and approved for use in food products by FDA in 1981.
The structure of aspartame is shown below.
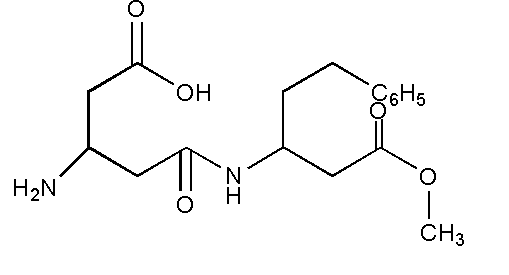
In the structure, it can be seen that the aspartame contains a total four functional groups. The functional groups are defined as those atoms or groups present inside the molecule which have their own characteristics property.
The functional groups present are amine \[ - N{H_2}\], carboxylic acid –COOH, ester \[ - COOR\], amide \[ - CONR\]
Aspartame on hydrolysis gives methanol, phenylalanine and aspartic acid as a product where phenylalanine and aspartic acid are two amino acids. Thus, on hydrolysis aspartame produces a mixture of two amino acids.
So, the correct answer is Option A,D.
Note: Aspartame due to its property of sweetener provides 4 kcal of energy per gram. The quantity of aspartame required to produce a sweet taste is small enough that its caloric value is negligible.
Complete step by step answer:
Aspartame is an artificial sweetener which is a non-saccharides which is 200 times sweeter than the sucrose. It is commonly used as a sugar substitute added in food and beverages. Aspartame is a methyl ester of aspartic acid. Aspartame is a formed in 1965 and approved for use in food products by FDA in 1981.
The structure of aspartame is shown below.

In the structure, it can be seen that the aspartame contains a total four functional groups. The functional groups are defined as those atoms or groups present inside the molecule which have their own characteristics property.
The functional groups present are amine \[ - N{H_2}\], carboxylic acid –COOH, ester \[ - COOR\], amide \[ - CONR\]
Aspartame on hydrolysis gives methanol, phenylalanine and aspartic acid as a product where phenylalanine and aspartic acid are two amino acids. Thus, on hydrolysis aspartame produces a mixture of two amino acids.
So, the correct answer is Option A,D.
Note: Aspartame due to its property of sweetener provides 4 kcal of energy per gram. The quantity of aspartame required to produce a sweet taste is small enough that its caloric value is negligible.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




