
Aspartame, an artificial sweetener, is a peptide and has the following structure:
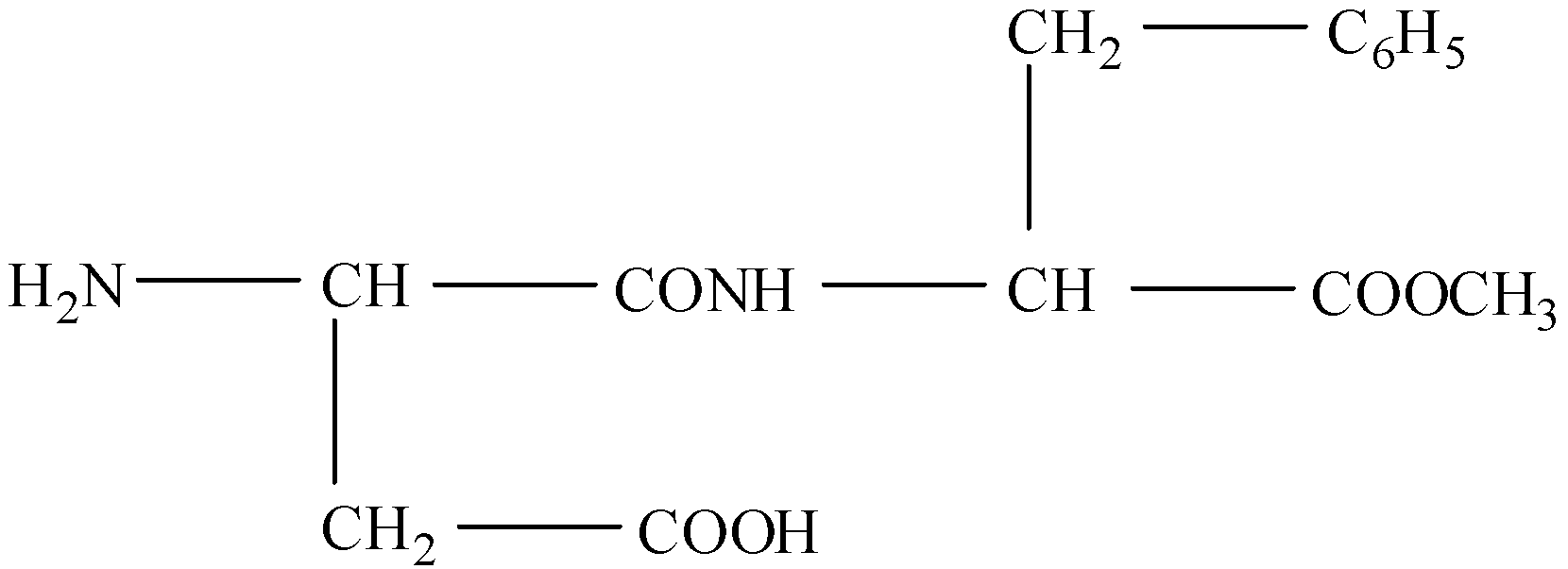
(I)- Identify the four functional groups
(II)- Write the zwitterionic structure
(III)- Write the structure of the amino acids obtained from the hydrolysis of aspartame.
(IV)- Which of the two amino acids is more hydrophobic?

Answer
533.1k+ views
Hint: Zwitterion is formed when the compound has both a basic and acidic group, then the hydrogen ion of the acidic group will shift towards the basic group. When the aspartame is hydrolyzed then the amide bond will break into an acidic and basic group. The part that contains more hydrocarbon will be more hydrophobic.
Complete answer:
The given compound is aspartame also known as aspartamine is an artificial sweetener.
(I)- The given structure is:
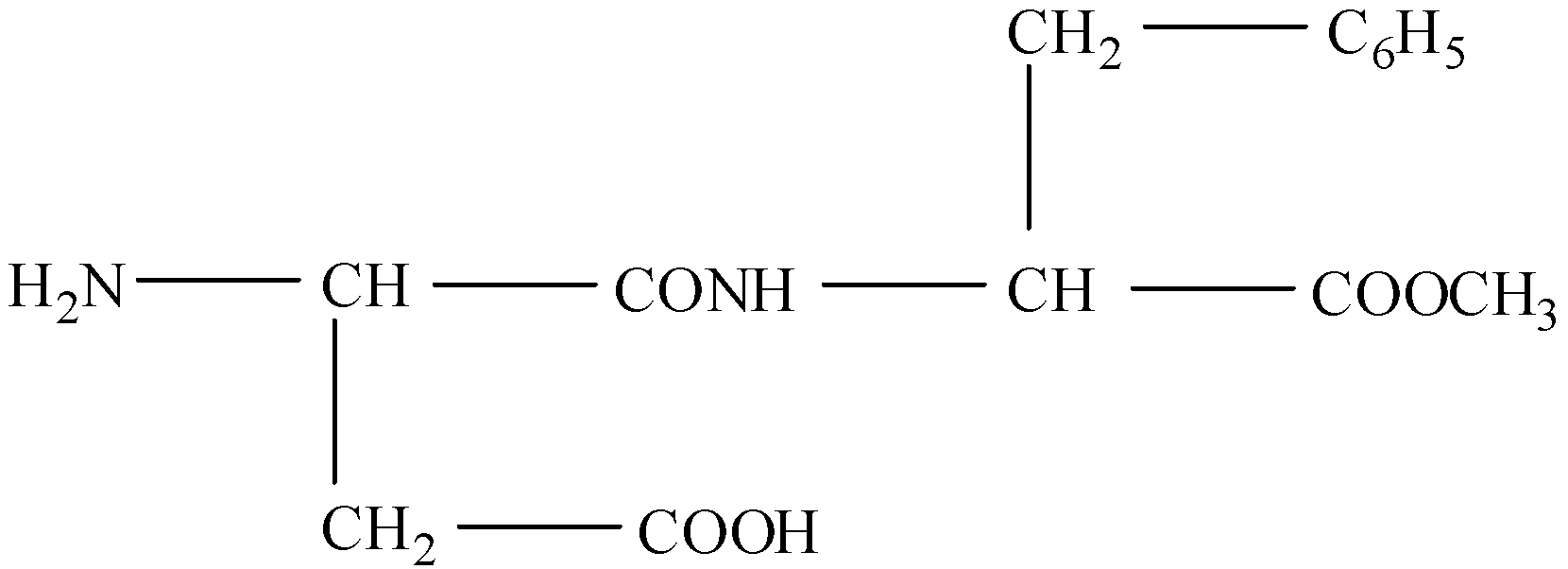
There are four functional groups in this compound. These are:
(a)- $-N{{H}_{2}}$ (amine)
(b)- -COOH (carboxylic acid)
(c)- -CONH (amide)
(d)- -COO- (ester)
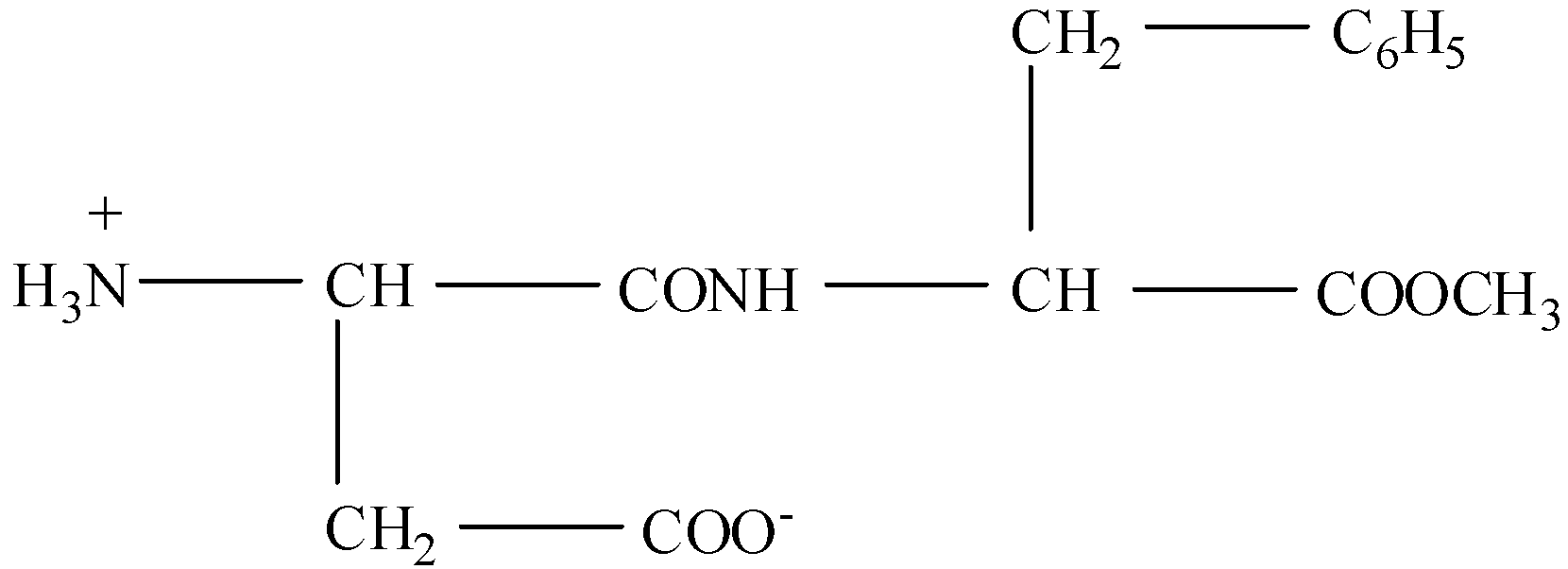
(II)- Zwitterion is formed when the compound has both a basic and acidic group, then the hydrogen ion of the acidic group will shift towards the basic group. The zwitterion in this compound will be:
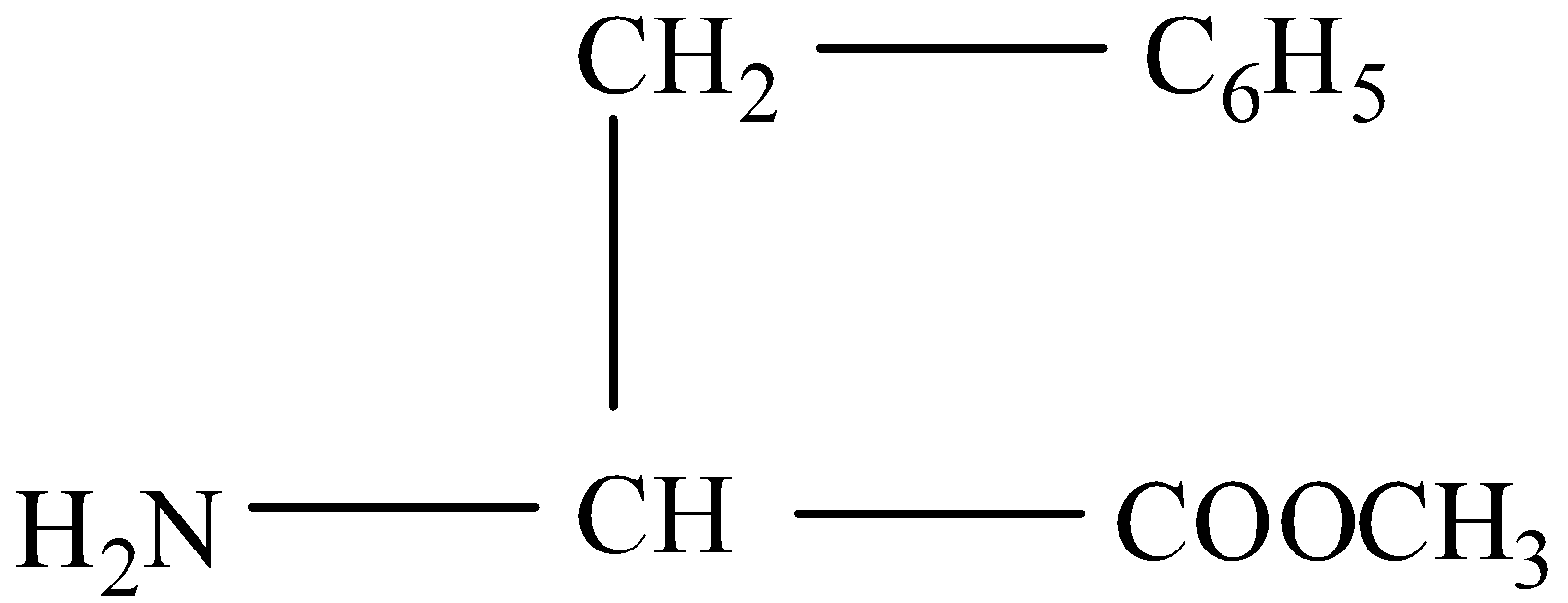
(III)- When the aspartame is hydrolyzed then the amide bond will break into an acidic and basic group. In the amide form –CONH- will be converted into one compound having a carboxylic group and the other having an amine group. The reaction is given below:
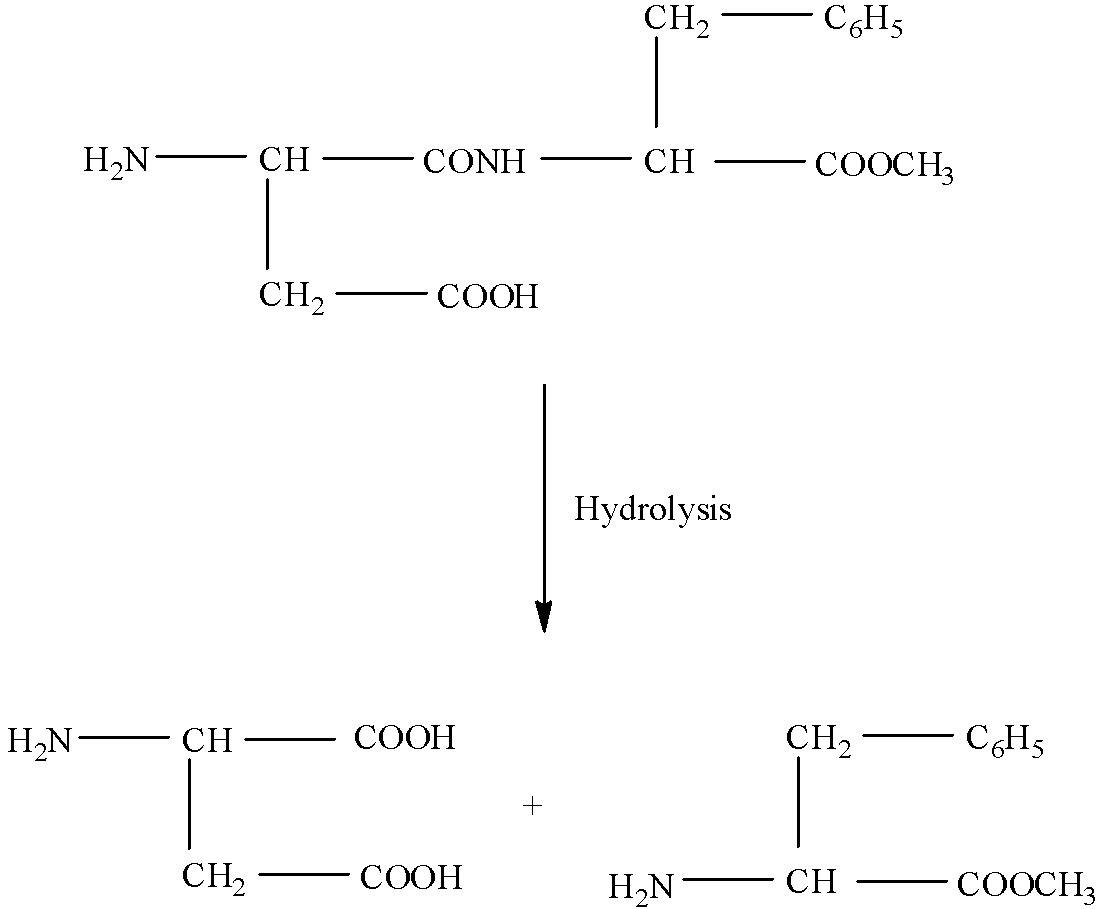
(IV)- In the given structure of the compound the part that contains more hydrocarbon part will be more hydrophobic. In this, the part in which the benzyl group is present will be more hydrophobic.
Note:
Aspartame is a peptide because it is formed by the formation of the peptide bond, and it is an amino acid because it has an amine group as well as a carboxylic acid group.
Complete answer:
The given compound is aspartame also known as aspartamine is an artificial sweetener.
(I)- The given structure is:

There are four functional groups in this compound. These are:
(a)- $-N{{H}_{2}}$ (amine)
(b)- -COOH (carboxylic acid)
(c)- -CONH (amide)
(d)- -COO- (ester)
(II)- Zwitterion is formed when the compound has both a basic and acidic group, then the hydrogen ion of the acidic group will shift towards the basic group. The zwitterion in this compound will be:

(III)- When the aspartame is hydrolyzed then the amide bond will break into an acidic and basic group. In the amide form –CONH- will be converted into one compound having a carboxylic group and the other having an amine group. The reaction is given below:

(IV)- In the given structure of the compound the part that contains more hydrocarbon part will be more hydrophobic. In this, the part in which the benzyl group is present will be more hydrophobic.

Note:
Aspartame is a peptide because it is formed by the formation of the peptide bond, and it is an amino acid because it has an amine group as well as a carboxylic acid group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




