
Asmi and Susmi have taken two containers of the same volume attached with two narrow tubes containing small amounts of coloured liquid as shown in the given figures. One container is filled with ideal gas and the other one is filled with real gas. If the number of moles of gases present in two containers are the same and their temperatures are also same, identify the container which contains ideal gas.
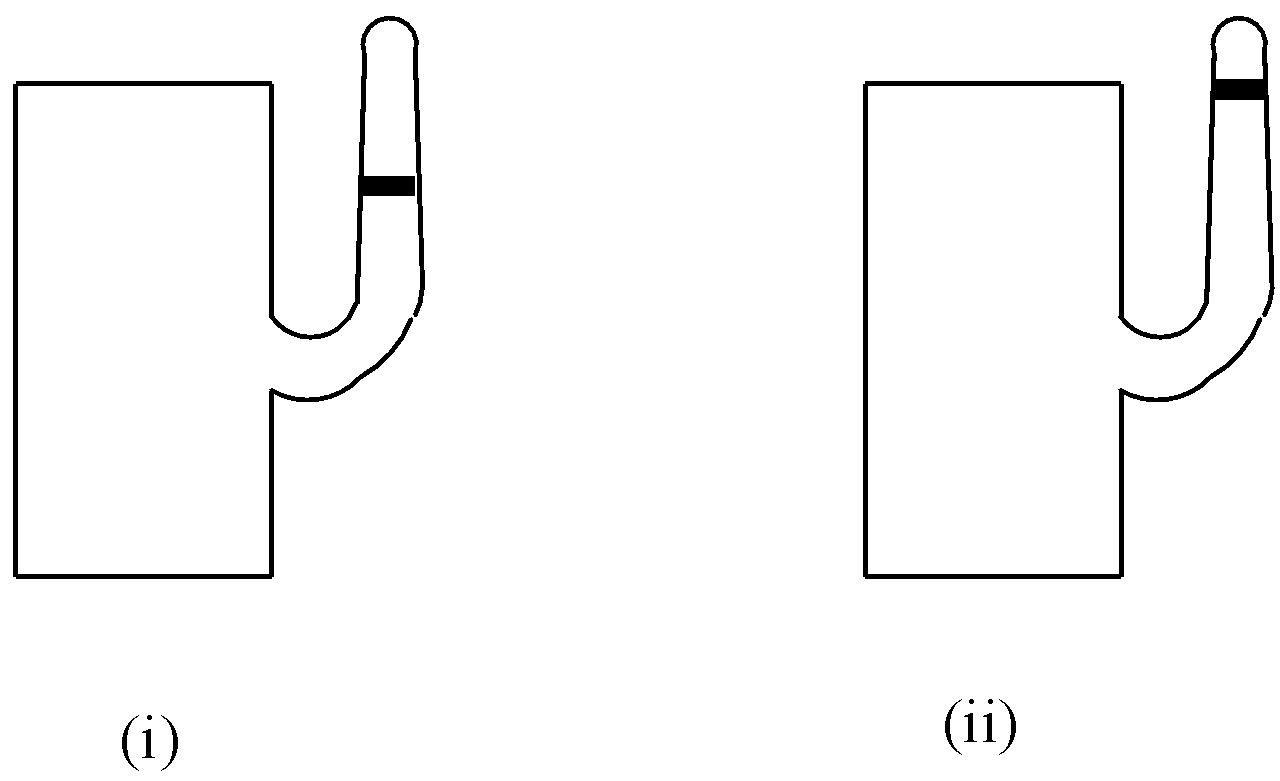
A. (i)
B. (ii)
C. both (i) and (ii)
D. cant be predicted

Answer
542.4k+ views
Hint: Ideal gas can be said to have no definite volume, and it also has no attractive forces present. So, the container which occupies more volume should be ideal gas.
Complete answer:
In order to answer our question we need to learn about real gases and ideal gas properties. The features that a real gas possess in contrast to ideal gas are:
Actual volume of gas molecules is negligible in comparison to the total volume of the gas : All the gases are made up of extremely small particles called molecules dispersed throughout the container. These particles are so small that they are regarded as point masses As they are point masses, so the actual volume occupied by the gas molecules is negligible in comparison to the total volume of the gas.
No force of attraction between the gas molecules: As the distance between the gas molecules is very large, so it is assumed that there is no force of attraction between the gas molecules at ordinary temperature and pressure.
Particles of gas are in constant random motion: This assumption is supported by the fact that gases do not have a fixed shape because of their random motion.
Particles of gas collide with each other and with the walls of the container: Particles of gas move in the straight line with high velocities in all the possible directions. During this motion, they collide with each other and with the walls of the container in which gas is enclosed and even change direction upon collisions.
Now, let us come to the question. Real gases have a definite volume and there is attractive force present. So, ideal gas should consume more volume as there is no attractive force, and so, in the second container, ideal gas is present as it is larger in volume.
So, we obtain option B as the correct answer for the question.
Note:
It is to be noted that the collisions that occur in the gas molecules, when they are in motion, are perfectly elastic, hence there is no loss of energy.
Complete answer:
In order to answer our question we need to learn about real gases and ideal gas properties. The features that a real gas possess in contrast to ideal gas are:
Actual volume of gas molecules is negligible in comparison to the total volume of the gas : All the gases are made up of extremely small particles called molecules dispersed throughout the container. These particles are so small that they are regarded as point masses As they are point masses, so the actual volume occupied by the gas molecules is negligible in comparison to the total volume of the gas.
No force of attraction between the gas molecules: As the distance between the gas molecules is very large, so it is assumed that there is no force of attraction between the gas molecules at ordinary temperature and pressure.
Particles of gas are in constant random motion: This assumption is supported by the fact that gases do not have a fixed shape because of their random motion.
Particles of gas collide with each other and with the walls of the container: Particles of gas move in the straight line with high velocities in all the possible directions. During this motion, they collide with each other and with the walls of the container in which gas is enclosed and even change direction upon collisions.
Now, let us come to the question. Real gases have a definite volume and there is attractive force present. So, ideal gas should consume more volume as there is no attractive force, and so, in the second container, ideal gas is present as it is larger in volume.
So, we obtain option B as the correct answer for the question.
Note:
It is to be noted that the collisions that occur in the gas molecules, when they are in motion, are perfectly elastic, hence there is no loss of energy.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




