
Arrange the various resonating structure of formic acid in their stability:
A.
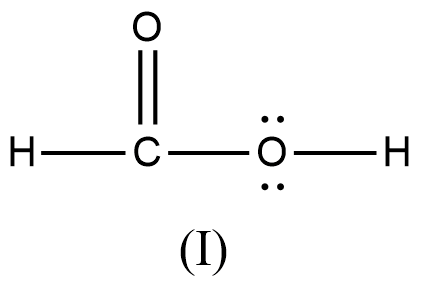
B.
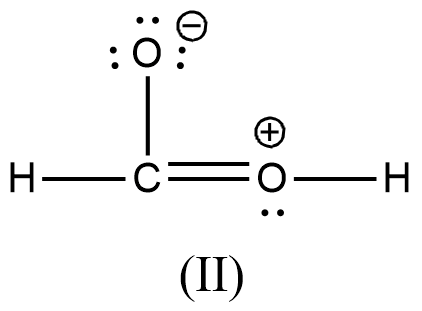
C.
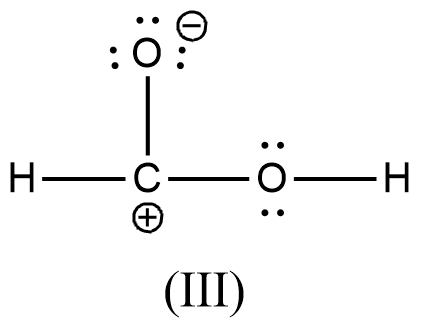
D.
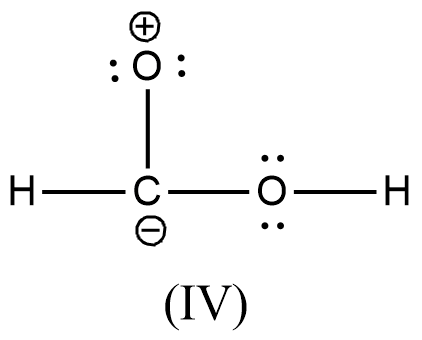




Answer
509.1k+ views
Hint: The molecular formula of formic acid is HCOOH. Due to the presence of the lone pair of electrons and pi-bond the formic acid is going to show the resonance structures. The delocalization of the lone pair of electrons and the pi bond makes the molecule show the resonating structures.
Complete answer:
- In the question it is asked to arrange the resonating structures of given formic acid as per their stability.
- The delocalization of the free electrons (lone pair or pi electrons) is the main cause of the resonance in the molecules.
- If the molecule is going to exhibit the resonance structures, it means that the molecule is going to exhibit stability.
- In the resonance structures, the most electronegative element is going to carry a negative charge and the most electropositive element is going to carry a positive charge on it.
- In the resonance structures the same charges should be present on the neighbor atoms and opposite charges are going to be spread widely throughout the molecule.
- So, we can write the stability order of the resonating structures of the formic acid as given below.
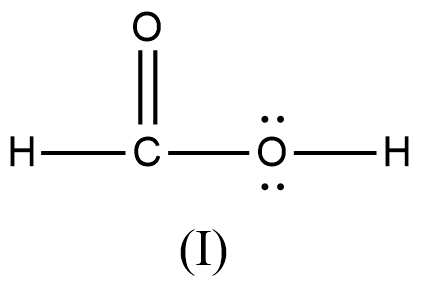 >
>
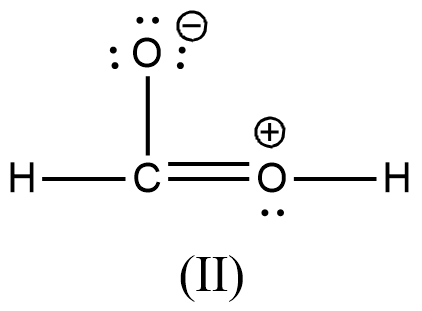 >
>
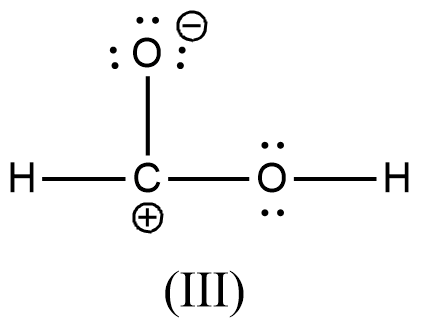 >
>
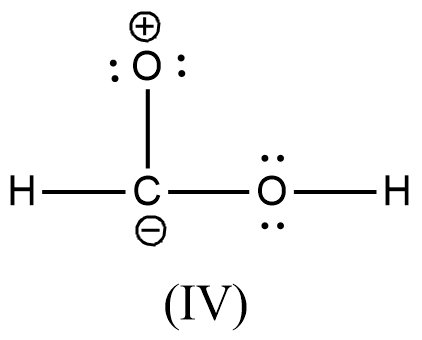
- We can write like this also, (I) > (II) > (III) > (IV)
Note:
Mostly the atom whose electronegativity is very high is going to carry a negative charge while the atom which is going to have less electronegativity is going to carry a positive charge in it in the resonance structures.
Complete answer:
- In the question it is asked to arrange the resonating structures of given formic acid as per their stability.
- The delocalization of the free electrons (lone pair or pi electrons) is the main cause of the resonance in the molecules.
- If the molecule is going to exhibit the resonance structures, it means that the molecule is going to exhibit stability.
- In the resonance structures, the most electronegative element is going to carry a negative charge and the most electropositive element is going to carry a positive charge on it.
- In the resonance structures the same charges should be present on the neighbor atoms and opposite charges are going to be spread widely throughout the molecule.
- So, we can write the stability order of the resonating structures of the formic acid as given below.




- We can write like this also, (I) > (II) > (III) > (IV)
Note:
Mostly the atom whose electronegativity is very high is going to carry a negative charge while the atom which is going to have less electronegativity is going to carry a positive charge in it in the resonance structures.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




