
Arrange the reactions given below in proper sequence for the conversion of ethane to methane.
(a) Treatment with sodium hydroxide (b) Treatment with aqueous $KOH$ (c) Treatment with $NaOH + CaO$ (d) Halogenation (e) Reaction with acidified ${K_2}C{r_2}{O_7}$
(A) e a d b c
(B) d b e a c
(C) e c b a d
(D) d e a b c
Answer
581.4k+ views
Hint: This reaction involves the oxidation of the hydroxyl group to the carboxylic acid group. The mixture of NaOH and CaO is called soda-lime and it is famous for its use in decarboxylation reactions.
Complete step by step solution:
The complete process of conversion of ethane to methane is given below.
1) Conversion of ethane to chloroethane(Halogenation):
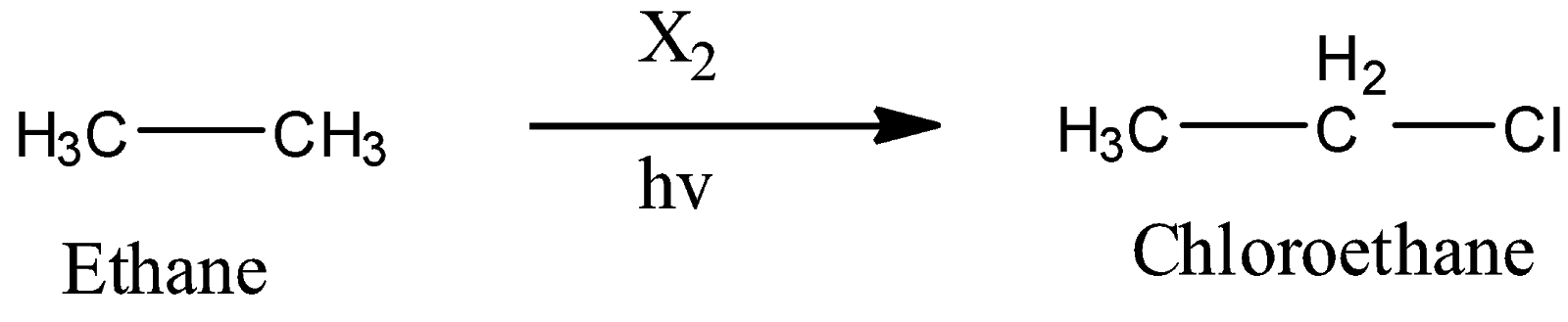
Here, we can see that the halogenation of ethane is carried out in presence of light and the halogen gas. This reaction goes by free radical mechanism and gives chloroethane.
2) Treatment with aqueous $KOH$:
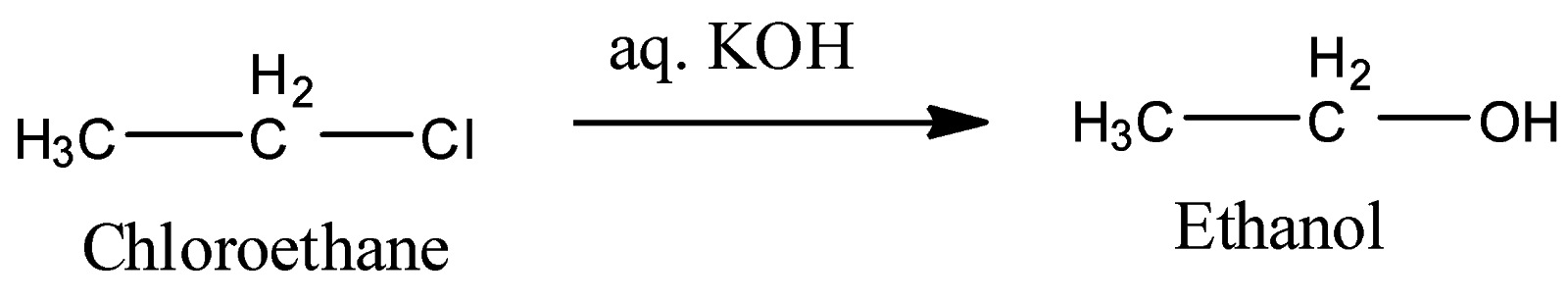
Here, the substitution reaction occurs.$–Cl$ gets replaced by $–OH$ group, it gives ethanol as the final product. This reaction goes by the ${S_N}1$ mechanism as the solvent is polar and the substrate is a primary halide.
3) Treatment with acidified potassium dichromate:
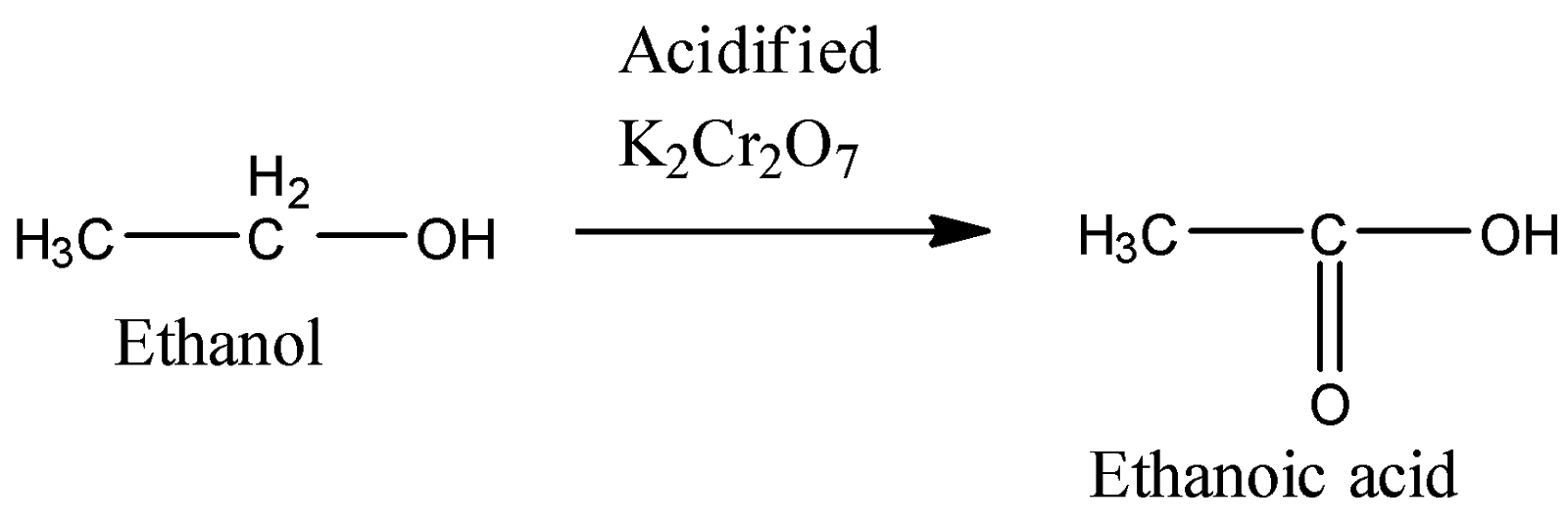
Here, the hydroxyl group gets oxidized with the oxidizing agent chromic acid $({H_2}C{r_2}{O_7})$ which is formed in-situ. Thus, we obtain ethanoic acid.
4) Treatment with $NaOH$:
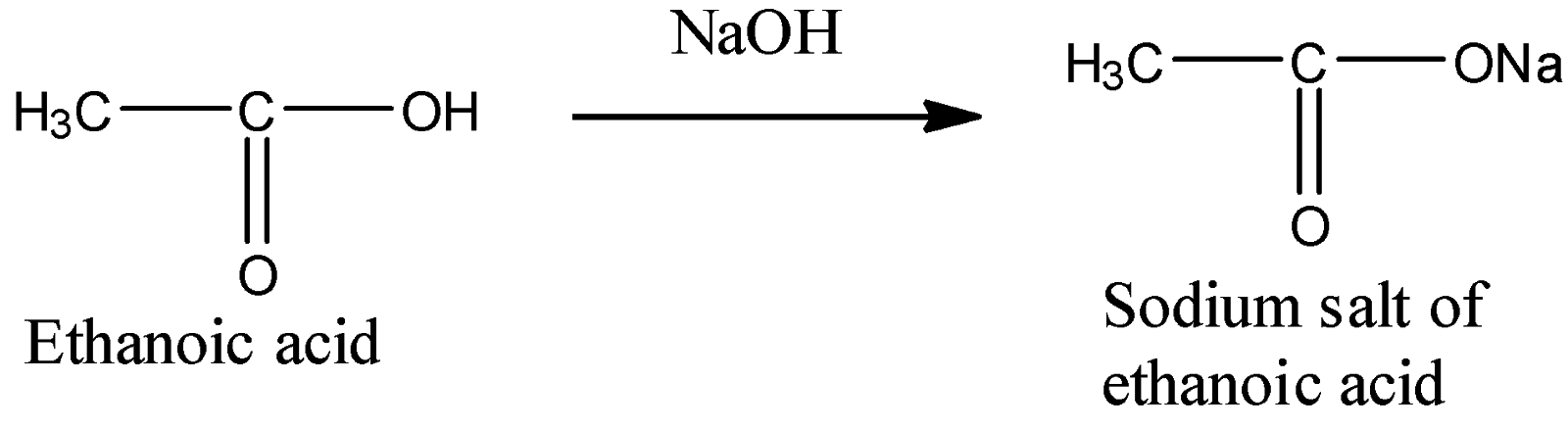
This is just an acid-base type of reaction in which salt and water get formed. So, we get a sodium salt of ethanoic acid as a product.
5) Reaction with $NaOH + CaO$
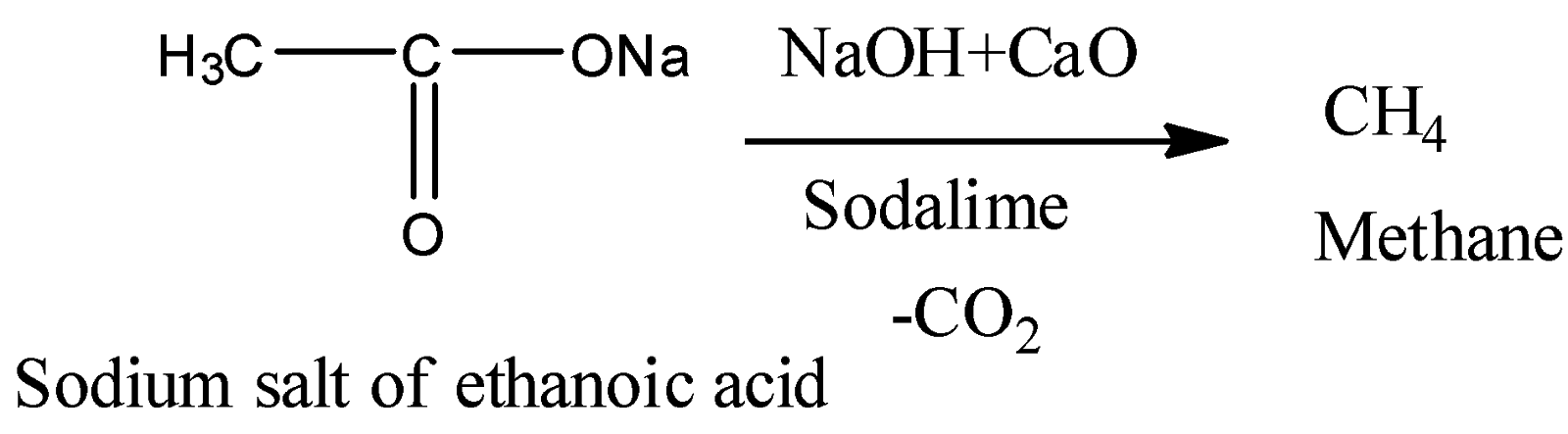
Here, we can see that the carbon dioxide gas is removed upon the reaction of the sodium salt of acid with $NaOH$ and $CaO$. The mixture of $NaOH$ and $CaO$ is also called soda lime. So, this is a decarboxylation reaction. It gives methane as an end product.
Thus, we can say that the correct order of the reaction is d b e a c
Therefore, the correct answer is (B).
Note: With this reaction order, we can remove one carbon from any straight-chain hydrocarbon compound. Note that acid directly cannot give a decarboxylation reaction. It is necessary to convert it into its salt first and then its reaction with soda lime converts it to the alkane having one less carbon.
Complete step by step solution:
The complete process of conversion of ethane to methane is given below.
1) Conversion of ethane to chloroethane(Halogenation):
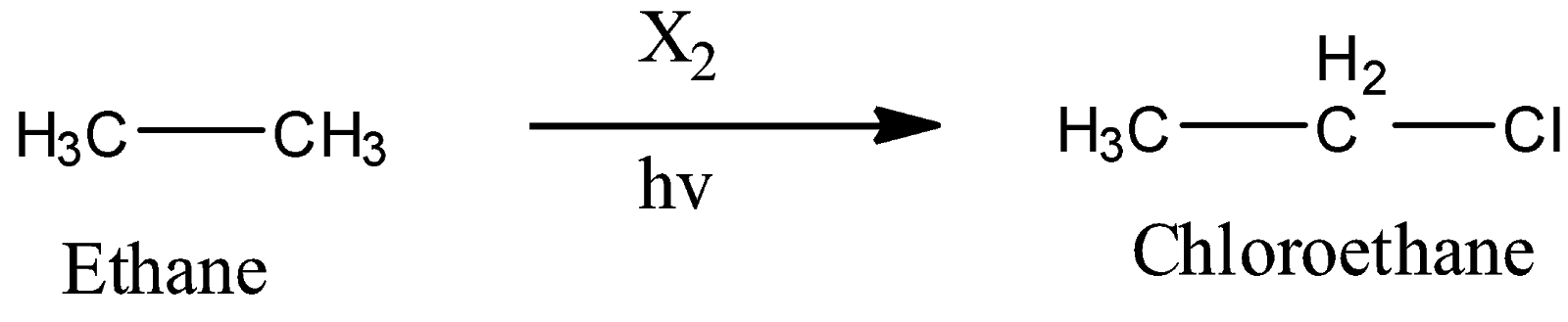
Here, we can see that the halogenation of ethane is carried out in presence of light and the halogen gas. This reaction goes by free radical mechanism and gives chloroethane.
2) Treatment with aqueous $KOH$:
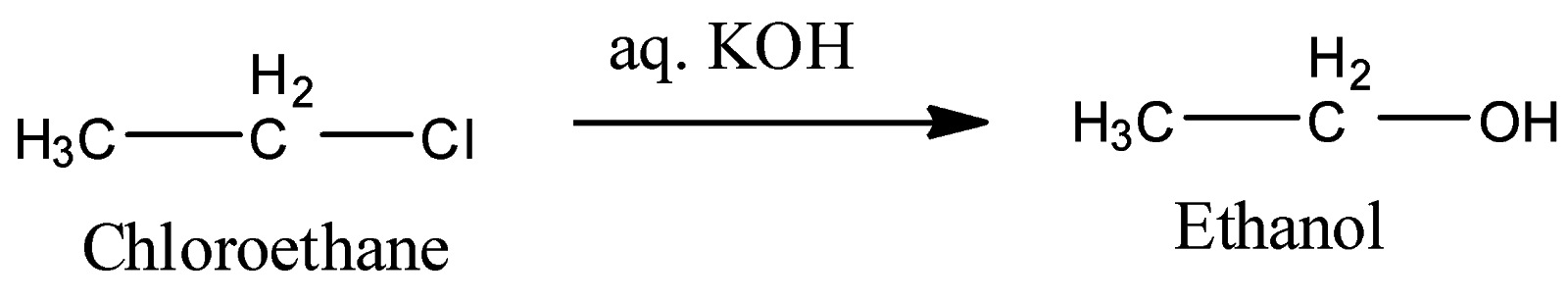
Here, the substitution reaction occurs.$–Cl$ gets replaced by $–OH$ group, it gives ethanol as the final product. This reaction goes by the ${S_N}1$ mechanism as the solvent is polar and the substrate is a primary halide.
3) Treatment with acidified potassium dichromate:

Here, the hydroxyl group gets oxidized with the oxidizing agent chromic acid $({H_2}C{r_2}{O_7})$ which is formed in-situ. Thus, we obtain ethanoic acid.
4) Treatment with $NaOH$:

This is just an acid-base type of reaction in which salt and water get formed. So, we get a sodium salt of ethanoic acid as a product.
5) Reaction with $NaOH + CaO$

Here, we can see that the carbon dioxide gas is removed upon the reaction of the sodium salt of acid with $NaOH$ and $CaO$. The mixture of $NaOH$ and $CaO$ is also called soda lime. So, this is a decarboxylation reaction. It gives methane as an end product.
Thus, we can say that the correct order of the reaction is d b e a c
Therefore, the correct answer is (B).
Note: With this reaction order, we can remove one carbon from any straight-chain hydrocarbon compound. Note that acid directly cannot give a decarboxylation reaction. It is necessary to convert it into its salt first and then its reaction with soda lime converts it to the alkane having one less carbon.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




