
Arrange the following resonating structures according to decreasing order of stability
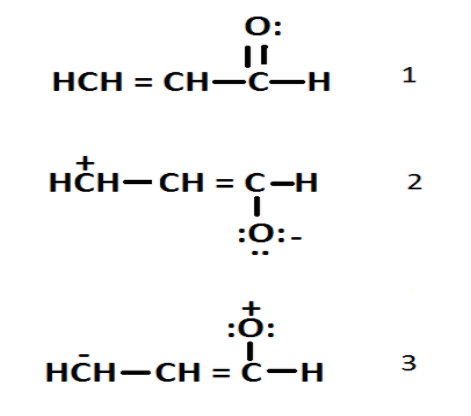
1. \[\;1{\text{ }} > {\text{ }}2{\text{ }} > {\text{ }}3\]
2. \[\;2{\text{ }} > {\text{ }}1{\text{ }} > {\text{ }}3\]
3. \[\;3{\text{ }} > {\text{ }}1{\text{ }} > {\text{ }}2\]
4. \[3{\text{ }} > {\text{ }}2{\text{ }} > {\text{ }}1\]
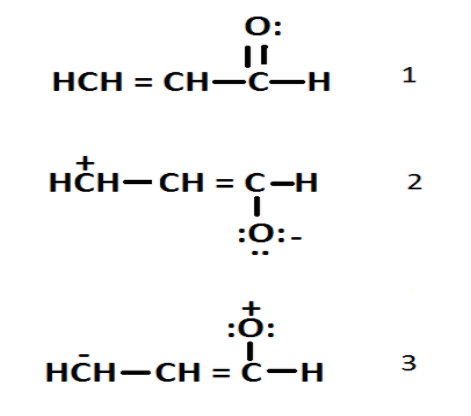
Answer
569.7k+ views
Hint: In the given question the stability of the resonating structures has been asked. Though the stability of acrolein. Acrolein is a colorless or yellow liquid with a specific odor and is used as a pesticide to control mollusks, weeds, bacteria, and algae.
Complete Step by step answer: We know that the stability of resonance increases with the increase in Number of covalent bonds, also the Number of atoms within an octet of electrons as exception in hydrogen because it has a duplex, also in the Separation of opposite charges followed with Dispersal of charge. So that a negative charge if any on a more electronegative atom, a positive charge if any on the more electropositive atom, increases the stability of the atom. Stability of the resonating structures depends on the following points they are as follows-First is that the equivalent resonating structure will Subsidize equally toward the right-hand side, but if resonating structures are not equivalent, they do so in course of their stability.
From the above structure we can say that the most stable structure is 1 then 2 and then 3.
Hence, option 1 is correct.
Note: The order of Stability of the resonating structure will be like that, electronegative element having incomplete octet and positive charge that the non-polar compounds will be on the right hand side. Non-polar resonating structure, Polar with Complete octet of their atoms has maximum no of Covalent bonds. If negative charge is present it should be a more electronegative element. Less charge separation is more stable than more charge separation.
Complete Step by step answer: We know that the stability of resonance increases with the increase in Number of covalent bonds, also the Number of atoms within an octet of electrons as exception in hydrogen because it has a duplex, also in the Separation of opposite charges followed with Dispersal of charge. So that a negative charge if any on a more electronegative atom, a positive charge if any on the more electropositive atom, increases the stability of the atom. Stability of the resonating structures depends on the following points they are as follows-First is that the equivalent resonating structure will Subsidize equally toward the right-hand side, but if resonating structures are not equivalent, they do so in course of their stability.
From the above structure we can say that the most stable structure is 1 then 2 and then 3.
Hence, option 1 is correct.
Note: The order of Stability of the resonating structure will be like that, electronegative element having incomplete octet and positive charge that the non-polar compounds will be on the right hand side. Non-polar resonating structure, Polar with Complete octet of their atoms has maximum no of Covalent bonds. If negative charge is present it should be a more electronegative element. Less charge separation is more stable than more charge separation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




