
Arrange the following in increasing order of acidic strength.
(i) $ClC{{H}_{2}}COOH,\text{ }C{{H}_{2}}ClC{{H}_{2}}COOH,\text{ }FC{{H}_{2}}COOH,\text{ }C{{H}_{3}}COOH$
(ii) $C{{H}_{3}}C{{H}_{2}}CH\left( Br \right)COOH,\text{ }C{{H}_{3}}CH\left( Br \right)C{{H}_{2}}COOH,\text{ }{{\left( C{{H}_{3}} \right)}_{2}}CHCOOH,\text{ }C{{H}_{3}}C{{H}_{2}}C{{H}_{2}}COOH$
Answer
572.1k+ views
Hint: +I effect and –I effect are going to play a role in finding the acidity of the carboxylic acid. +I effect decreases the acidity of the carboxylic acid and –I effect increases the acidity of the carboxylic acids.
Complete step by step answer:
- In the question they have given a few carboxylic acids and asked to arrange them in the increasing order of acidity.
(i) The given carboxylic acids are as follows.
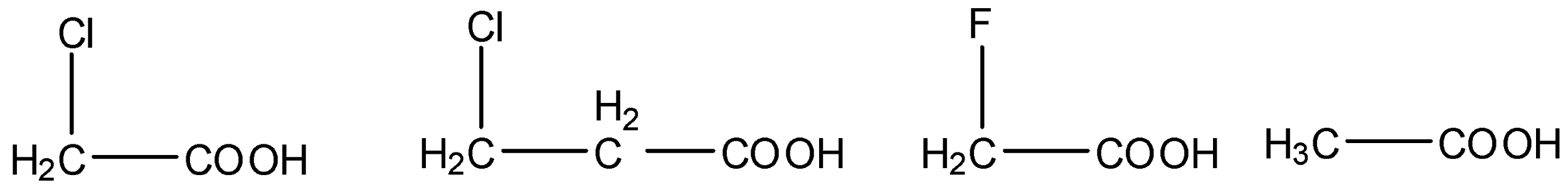
- We know that electron withdrawing groups are called –I effect groups. Electron withdrawing groups withdraw the electrons from the carboxylic groups and make the hydrogen to release freely into the solution.
- Means electron withdrawing groups increases the acidity of the carboxylic acids.
- We know that fluorine is more electronegative than chlorine then the carboxylic acids which contain fluorine are highly acidic in nature.
- Then the acidity of the fluoro acetic acid is high when compared to chloro acetic acid.
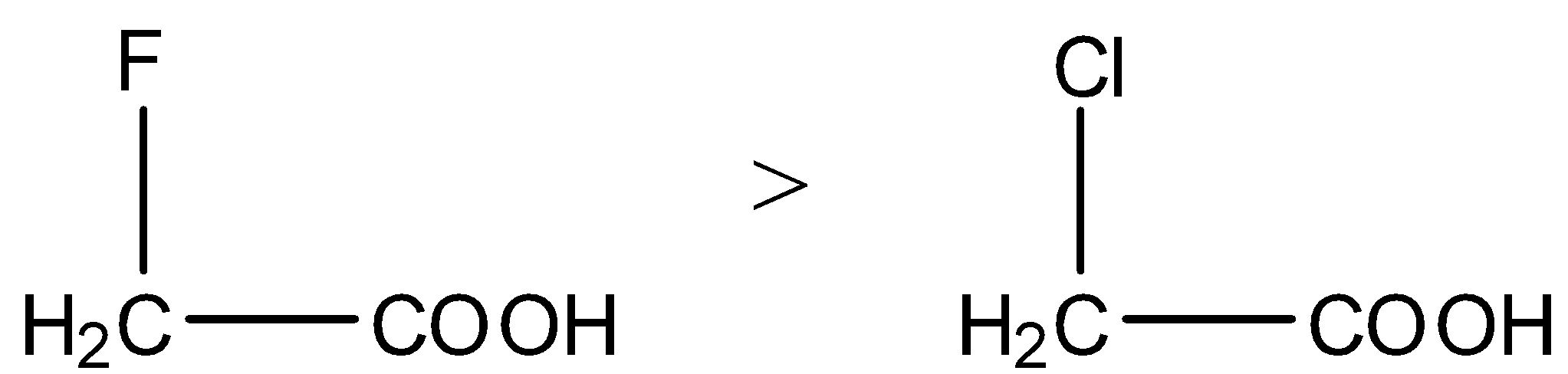
- As the distance of the electron withdrawing group from carboxylic acid increases then the strength of the carboxylic acid is going to decrease.
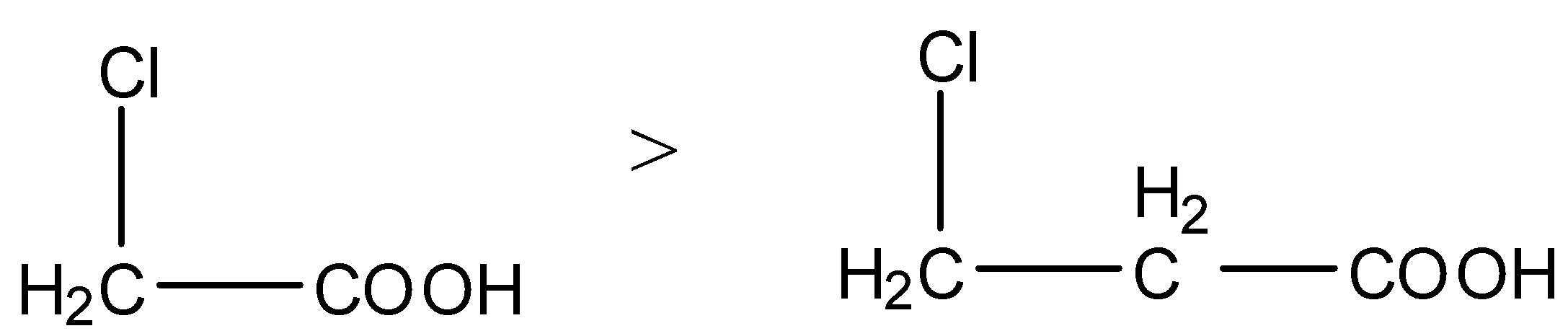
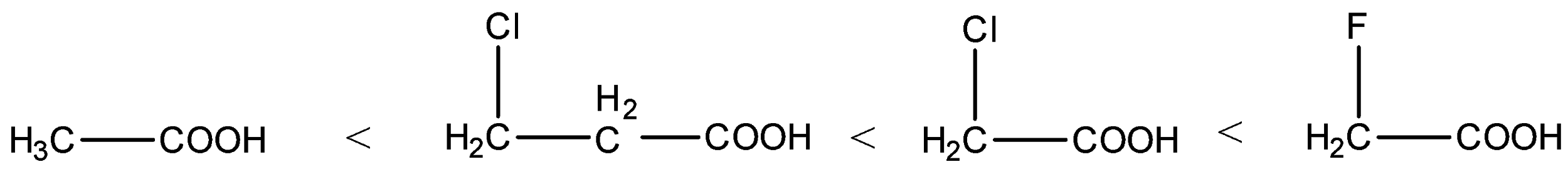
- Therefore the increasing acidity of the given carboxylic acids is as follows.
(ii) The given carboxylic acids are as follows.
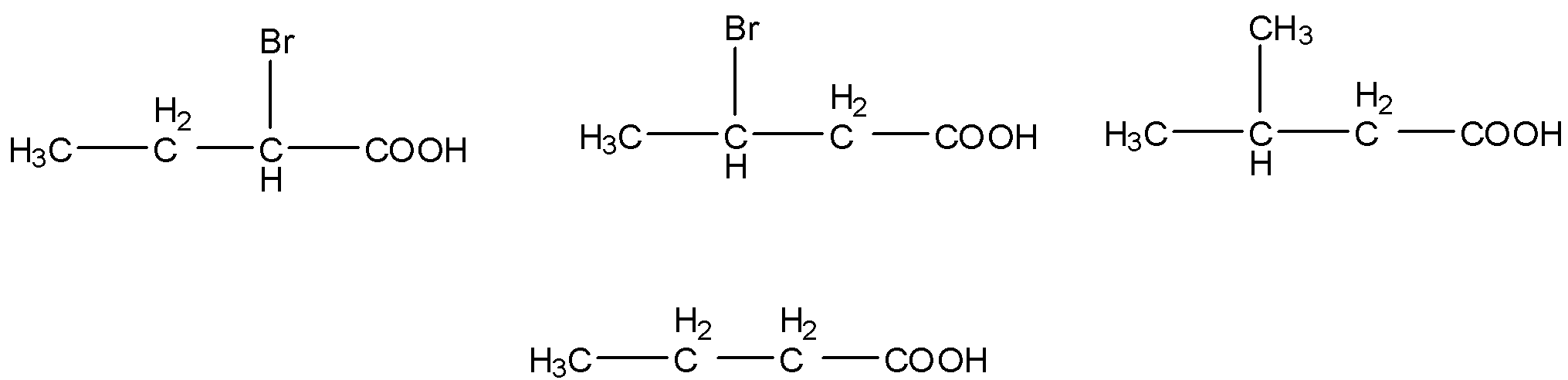
- Electron donating groups are called as +I groups, in the given carboxylic acids methyl groups act as electron donating groups and bromine atoms act as electron withdrawing groups (-I effect).
- We know that electron withdrawing groups increase the acidity of the carboxylic acids and electron donating groups decrease the acidity of the carboxylic acid molecules.
- Then the increasing the order of the acidity of the given carboxylic acids is as follows.
Note: The acidity of the carboxylic acids are going to depend on the electron donating groups and electron withdrawing groups attached to them. At the same time the distance between the electron withdrawing groups from the carboxylic acid also decides the acidity of the carboxylic acids.
Complete step by step answer:
- In the question they have given a few carboxylic acids and asked to arrange them in the increasing order of acidity.
(i) The given carboxylic acids are as follows.

- We know that electron withdrawing groups are called –I effect groups. Electron withdrawing groups withdraw the electrons from the carboxylic groups and make the hydrogen to release freely into the solution.
- Means electron withdrawing groups increases the acidity of the carboxylic acids.
- We know that fluorine is more electronegative than chlorine then the carboxylic acids which contain fluorine are highly acidic in nature.
- Then the acidity of the fluoro acetic acid is high when compared to chloro acetic acid.

- As the distance of the electron withdrawing group from carboxylic acid increases then the strength of the carboxylic acid is going to decrease.
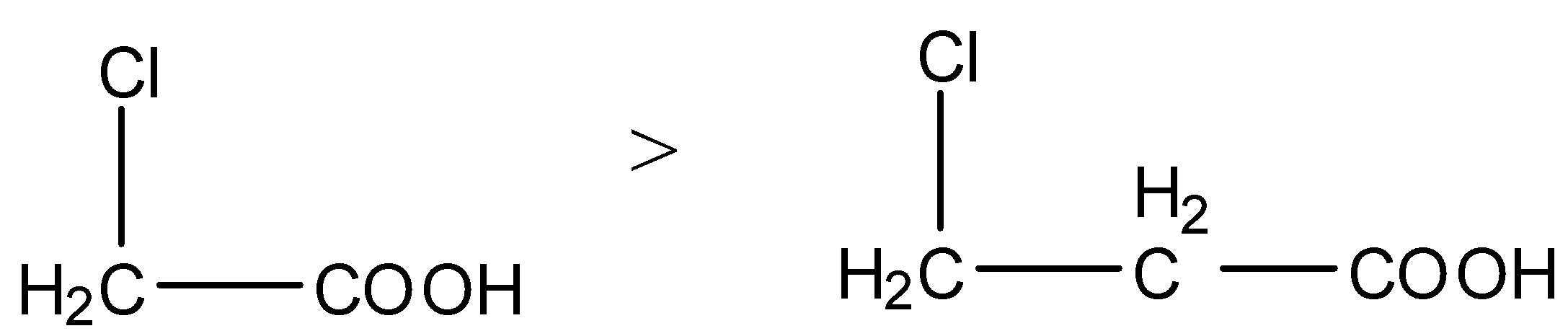
- Therefore the increasing acidity of the given carboxylic acids is as follows.

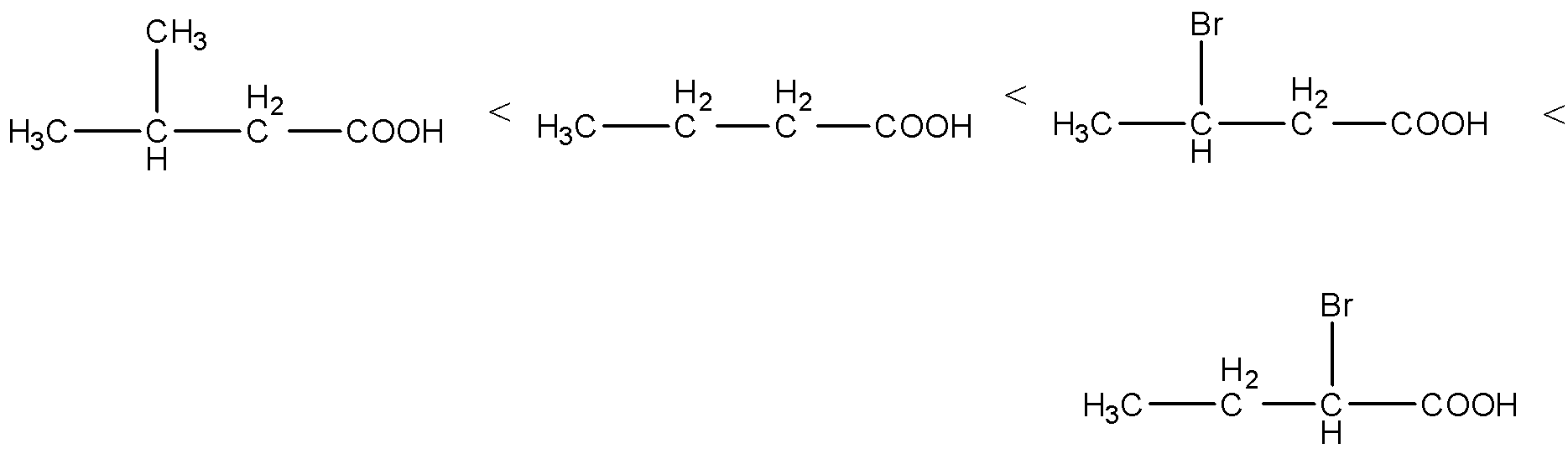
(ii) The given carboxylic acids are as follows.

- Electron donating groups are called as +I groups, in the given carboxylic acids methyl groups act as electron donating groups and bromine atoms act as electron withdrawing groups (-I effect).
- We know that electron withdrawing groups increase the acidity of the carboxylic acids and electron donating groups decrease the acidity of the carboxylic acid molecules.
- Then the increasing the order of the acidity of the given carboxylic acids is as follows.

Note: The acidity of the carboxylic acids are going to depend on the electron donating groups and electron withdrawing groups attached to them. At the same time the distance between the electron withdrawing groups from the carboxylic acid also decides the acidity of the carboxylic acids.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




