
Arrange the following in increasing order of stability.
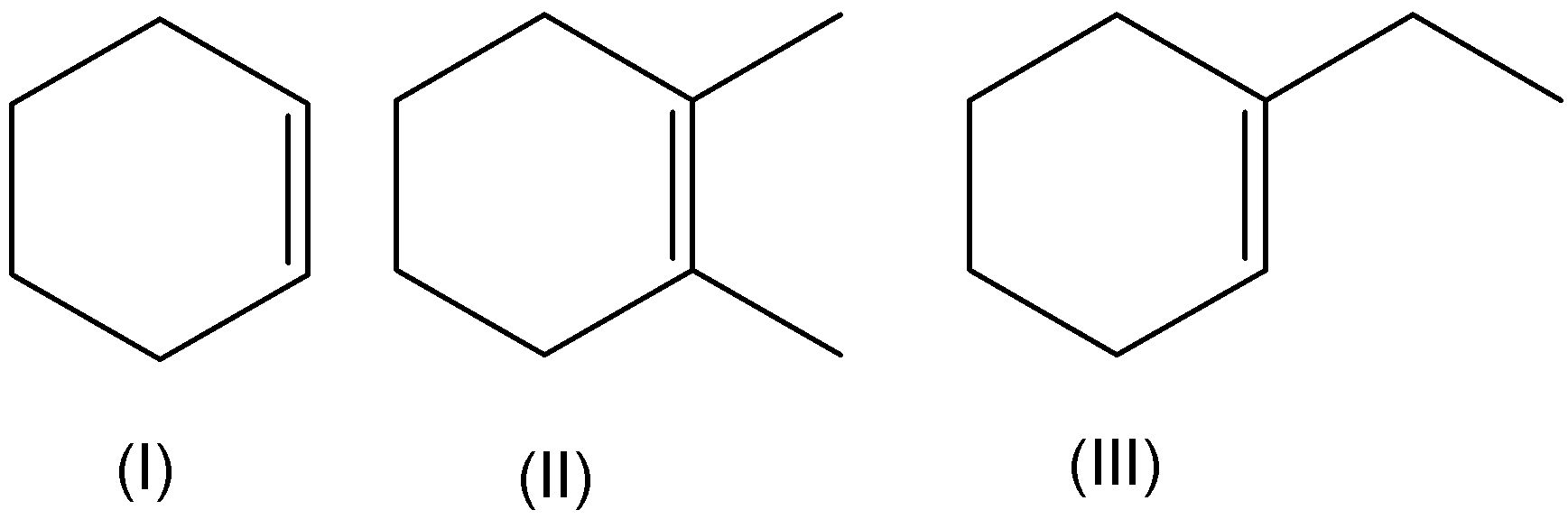
A.\[I < II < III\]
B.\[II < I < III\]
C.\[I < III < II\]
D.\[II < III < I\]

Answer
495.6k+ views
Hint: As we know that the stability of aromatic compounds depends upon so many factors. Resonance, oxidation, temperature etc will affect the stability of compounds. The lattice energy, solvation energy and energy of sublimation also affect the stability. The energy state mainly depends on the stability of the compound. When the compound is occupied in the lowest energy state then it becomes more stable. The intramolecular bond strength and intermolecular bond strength also depend on stability.
Complete answer:
The increasing order of stability is not equal to \[I < II < III\]. Hence, option (A) is incorrect.
Here, \[1 - \] ethylcyclohex\[ - 1 - \] ene is more stable than cyclohexene. And \[1,2 - \] dimethyl cyclohexene is the least stable compound. Because, \[1 - \] ethylcyclohex \[ - 1 - \] ene is a neutral compound. Thus, it is more stable. But cyclohexene and \[1,2 - \] dimethyl cyclohexene have a more stable resonating structure. Therefore, the increasing order of stability is \[II < I < III\]. Hence, option (B) is correct.
The increasing order of stability is not equal to \[I < III < II\]. Hence, option (C) is incorrect.
The increasing order of stability is not equal to \[II < III < I\]. Hence, option (D) is incorrect.
Hence, option (B) is correct.
Note:
The resonance will affect the stability. Because, the delocalization takes place by resonance. Then the total energy of the molecule will decrease since the electrons present in the molecule will occupy a large volume. The molecule becomes more stable by the delocalization of electrons because of the resonance. If the compound possesses the most stable resonance structure, then it could have less number of charges and the negative charge is located on the most electronegative atom.
Complete answer:
The increasing order of stability is not equal to \[I < II < III\]. Hence, option (A) is incorrect.
Here, \[1 - \] ethylcyclohex\[ - 1 - \] ene is more stable than cyclohexene. And \[1,2 - \] dimethyl cyclohexene is the least stable compound. Because, \[1 - \] ethylcyclohex \[ - 1 - \] ene is a neutral compound. Thus, it is more stable. But cyclohexene and \[1,2 - \] dimethyl cyclohexene have a more stable resonating structure. Therefore, the increasing order of stability is \[II < I < III\]. Hence, option (B) is correct.
The increasing order of stability is not equal to \[I < III < II\]. Hence, option (C) is incorrect.
The increasing order of stability is not equal to \[II < III < I\]. Hence, option (D) is incorrect.
Hence, option (B) is correct.
Note:
The resonance will affect the stability. Because, the delocalization takes place by resonance. Then the total energy of the molecule will decrease since the electrons present in the molecule will occupy a large volume. The molecule becomes more stable by the delocalization of electrons because of the resonance. If the compound possesses the most stable resonance structure, then it could have less number of charges and the negative charge is located on the most electronegative atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




