
How will you arrange the following in ascending order of acid strength and oxidizing capacity\[?\]$HCl{O_4},\,HCl{O_3},\,HCl{O_2},\,HClO$.
Answer
537.9k+ views
Hint: Acid strength is the tendency of an acid to dissociate into a proton and an anion and the acidic strength increases with the increase in stability of the corresponding conjugate base of the acid. Oxidizing property of a compound depends on its ability to give oxygen and hence order of oxidizing power is inversely proportional to the acidic strength.
Complete step by step answer:
Acid strength is the tendency of an acid to dissociate into a proton and an anion and the acidic strength increases with the increase in stability of the corresponding conjugate base of the acid.
So at first we need to find the conjugate bases of the acids given in the question and then arrange them in ascending order of their stability in order to find the order of acidic strength.
The acids given in the question are $HCl{O_4},\,HCl{O_3},\,HCl{O_2},\,HClO$ and their corresponding conjugate bases are:
$HCl{O_4}\xrightarrow{{ - {H^ + }}}Cl{O_4}^ - $
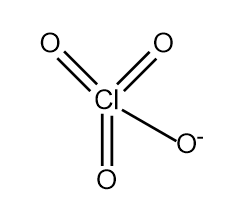
Which has four resonating structures.
$HCl{O_3}\xrightarrow{{ - {H^ + }}}Cl{O_3}^ - $
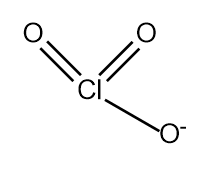
Which has three resonating structures.
$HCl{O_2}\xrightarrow{{ - {H^ + }}}Cl{O_2}^ - $
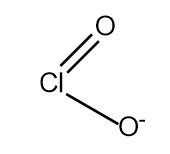
Which has two resonating structures.
$HClO\xrightarrow{{ - {H^ + }}}Cl{O^ - }$
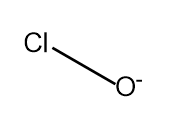
Which has no resonating structures.
Now, we know more the compound that has more number of resonating structures, that compound is stable.
Since $Cl{O_4}^ - ,\,Cl{O_3}^ - ,\,Cl{O_2}^ - ,\,Cl{O^ - }$ have $4,\,3,\,2,\,0$ resonating structures respectively. The stability order follows as: $Cl{O^ - } < Cl{O_2}^ - < Cl{O_3}^ - < Cl{O_4}^ - $.
Since acidic strength is directly proportional to the stability of the conjugate bases. Therefore the ascending order of the acidic strength follows the order:
$HClO < HCl{O_2} < HCl{O_3} < HCl{O_4}$.
Now, the oxidizing property of a compound depends on its ability to give oxygen and hence the order of oxidizing power is inversely proportional to the acidic strength.
Therefore the ascending order of oxidizing power or oxidizing capacity follows the order:
$HCl{O_4} < HCl{O_3} < HCl{O_2} < HClO$.
Note: You can also determine acidic strength of a compound as with increase in oxidation number of a particular halogen atom the acidic character of the corresponding oxoacid also increases. Also oxidizing property can be determined by the tendency of a compound to accept electrons and greater is the ability to accept electrons greater will be the oxidizing capacity.
Complete step by step answer:
Acid strength is the tendency of an acid to dissociate into a proton and an anion and the acidic strength increases with the increase in stability of the corresponding conjugate base of the acid.
So at first we need to find the conjugate bases of the acids given in the question and then arrange them in ascending order of their stability in order to find the order of acidic strength.
The acids given in the question are $HCl{O_4},\,HCl{O_3},\,HCl{O_2},\,HClO$ and their corresponding conjugate bases are:
$HCl{O_4}\xrightarrow{{ - {H^ + }}}Cl{O_4}^ - $

Which has four resonating structures.
$HCl{O_3}\xrightarrow{{ - {H^ + }}}Cl{O_3}^ - $

Which has three resonating structures.
$HCl{O_2}\xrightarrow{{ - {H^ + }}}Cl{O_2}^ - $

Which has two resonating structures.
$HClO\xrightarrow{{ - {H^ + }}}Cl{O^ - }$

Which has no resonating structures.
Now, we know more the compound that has more number of resonating structures, that compound is stable.
Since $Cl{O_4}^ - ,\,Cl{O_3}^ - ,\,Cl{O_2}^ - ,\,Cl{O^ - }$ have $4,\,3,\,2,\,0$ resonating structures respectively. The stability order follows as: $Cl{O^ - } < Cl{O_2}^ - < Cl{O_3}^ - < Cl{O_4}^ - $.
Since acidic strength is directly proportional to the stability of the conjugate bases. Therefore the ascending order of the acidic strength follows the order:
$HClO < HCl{O_2} < HCl{O_3} < HCl{O_4}$.
Now, the oxidizing property of a compound depends on its ability to give oxygen and hence the order of oxidizing power is inversely proportional to the acidic strength.
Therefore the ascending order of oxidizing power or oxidizing capacity follows the order:
$HCl{O_4} < HCl{O_3} < HCl{O_2} < HClO$.
Note: You can also determine acidic strength of a compound as with increase in oxidation number of a particular halogen atom the acidic character of the corresponding oxoacid also increases. Also oxidizing property can be determined by the tendency of a compound to accept electrons and greater is the ability to accept electrons greater will be the oxidizing capacity.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




