
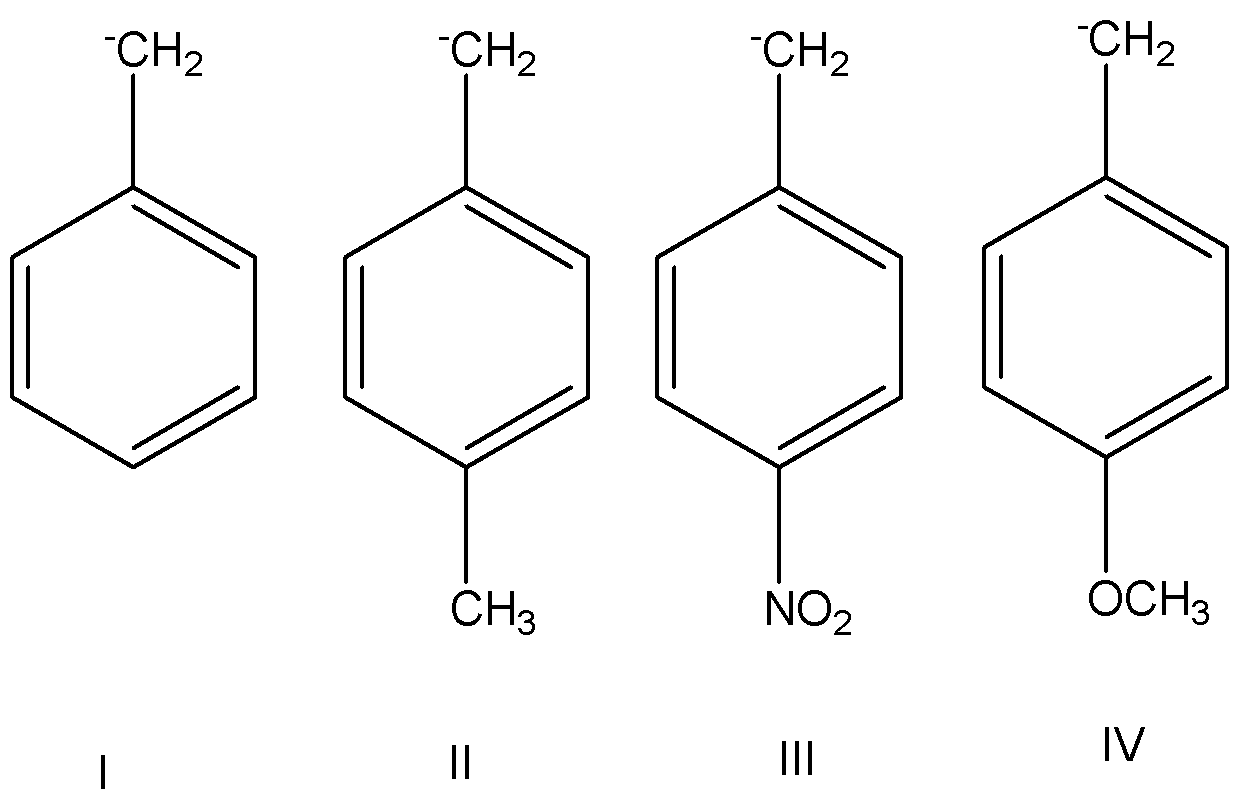
Arrange the following carbanions in decreasing order of stability:
A) III> I > IV > I
B) III > II > I > IV
C) I > III > II > IV
D) III > I > II > IV

Answer
516.3k+ views
Hint: We have to know that in organic chemistry stability of molecules and ions are very important. Carbon ions are classified as two types. There are carbocation and carbanion. The carbon atom which has a positive charge is called carbocation. The carbon atom which has a negative charge is called carbocation.
Complete answer:
We must have to know that electron displacement is the one of the reasons for the stability of molecules and ions in organic chemistry.
The given organic structure are
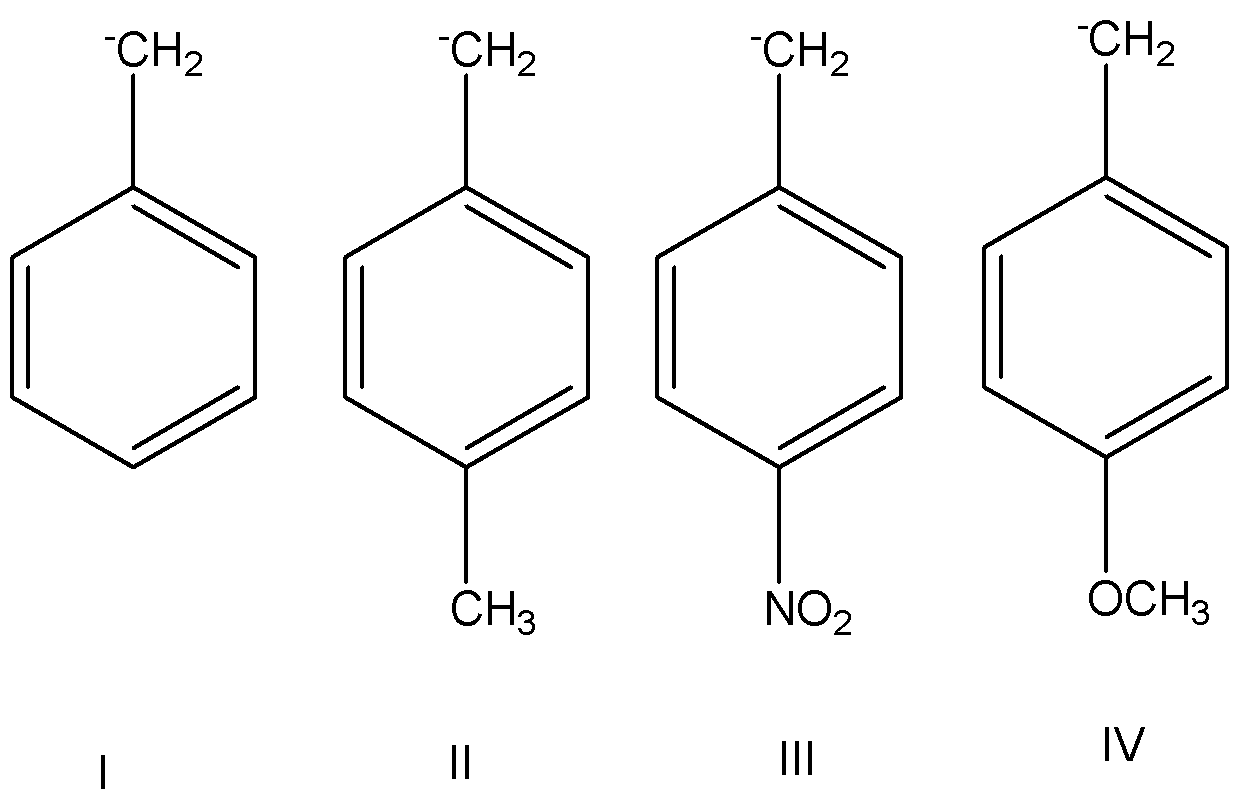
The organic structure having effect given are below:
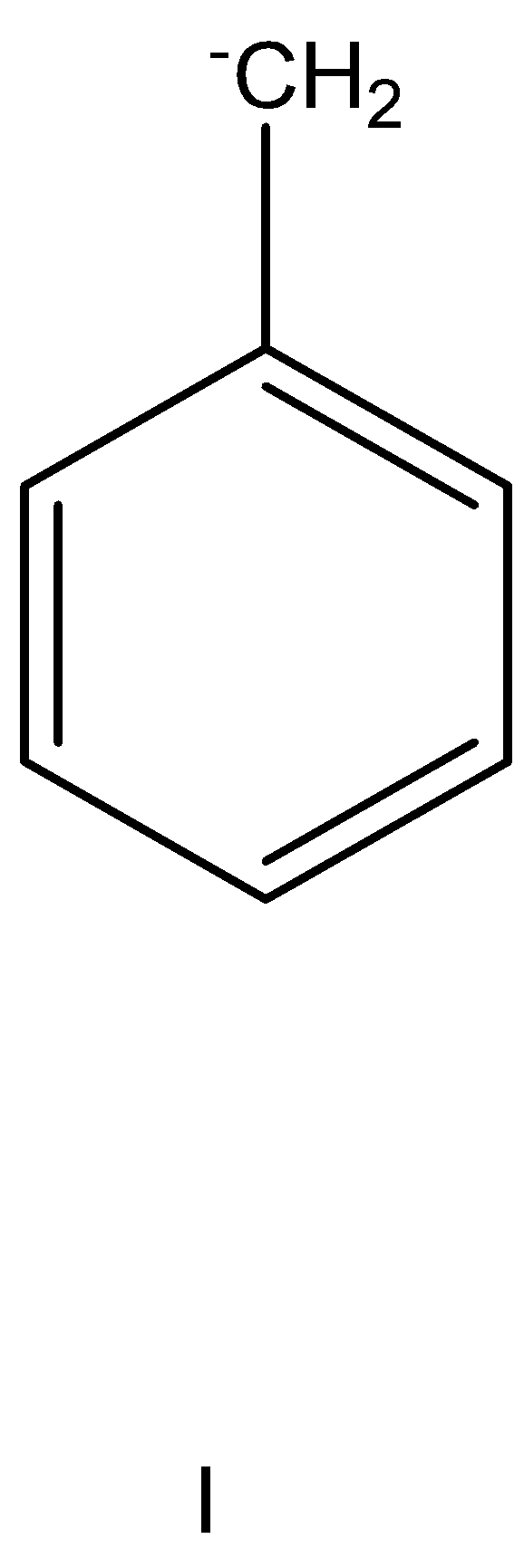 having no effect.
having no effect.
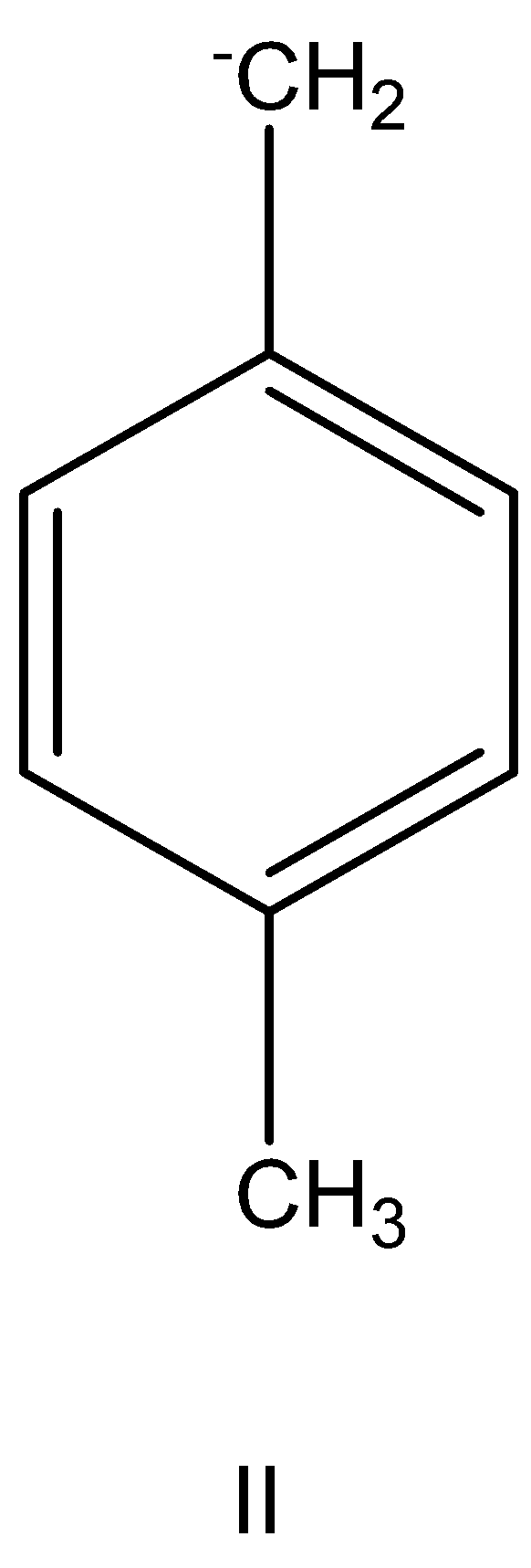 having +I and +HC effect.
having +I and +HC effect.
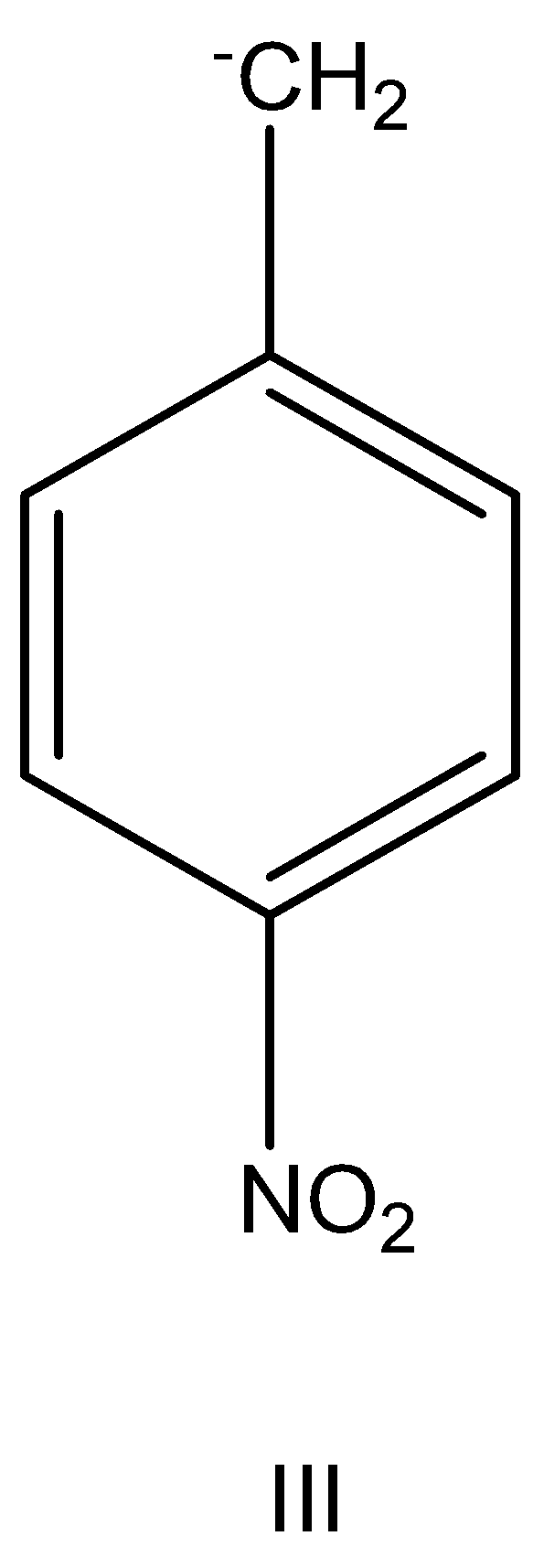 having –M effect.
having –M effect.
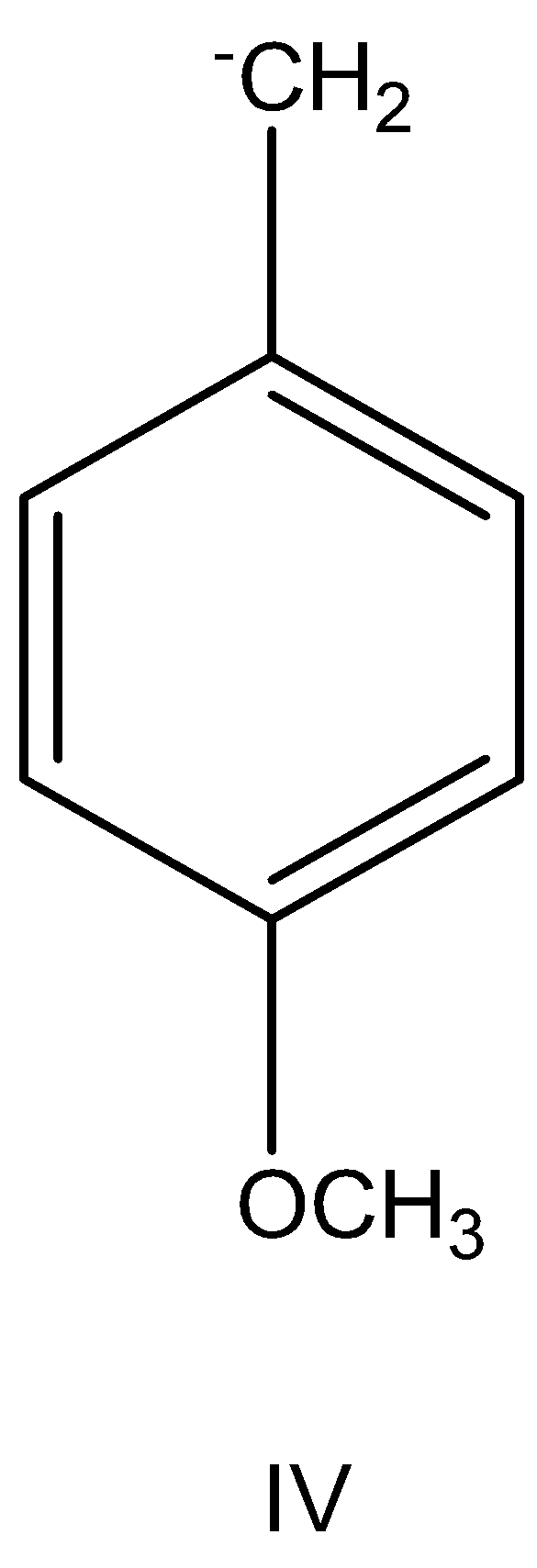 having +Meffect.
having +Meffect.
Hence, option D is correct because decreasing order of stability III > I > II > IV
Note:
We have to remember that the inductive effect, electromeric effect, resonance or mesomeric effect and hyper conjugate effect play an important role for electron displacement. Inductive effect is a permanent effect. Withdrawing or donating the electron density of the carbon atom by the neighbouring group or atom in that carbon atom in the molecule is known as an inductive effect. It is divided into two types. There are +I and –I effects. Electromeric effect is due to the movement of pi electrons. It is divided into two types. There are +E and –E effects. It is due to the direction of pi electron movement. Resonance or mesomeric effect means organic compounds which can be represented by more than one structure. It is divided into two types. There are +M and –M effects. It is due to differ only in the position of bonding and lone pair of electrons. Hyper conjugate effect is due to delocalisation of sigma electrons in molecules. It is divided into two types. There are +HC and –HC effects.
Complete answer:
We must have to know that electron displacement is the one of the reasons for the stability of molecules and ions in organic chemistry.
The given organic structure are

The organic structure having effect given are below:




Hence, option D is correct because decreasing order of stability III > I > II > IV
Note:
We have to remember that the inductive effect, electromeric effect, resonance or mesomeric effect and hyper conjugate effect play an important role for electron displacement. Inductive effect is a permanent effect. Withdrawing or donating the electron density of the carbon atom by the neighbouring group or atom in that carbon atom in the molecule is known as an inductive effect. It is divided into two types. There are +I and –I effects. Electromeric effect is due to the movement of pi electrons. It is divided into two types. There are +E and –E effects. It is due to the direction of pi electron movement. Resonance or mesomeric effect means organic compounds which can be represented by more than one structure. It is divided into two types. There are +M and –M effects. It is due to differ only in the position of bonding and lone pair of electrons. Hyper conjugate effect is due to delocalisation of sigma electrons in molecules. It is divided into two types. There are +HC and –HC effects.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




