
What are the shapes of $PC{l_4}^ + $ , $PC{l_4}^ - $ and $AsC{l_5}$ ?
A. Square planar, tetrahedral and see-saw
B. Tetrahedral, see-saw and trigonal bipyramidal
C. Tetrahedral, square planar and pentagonal bipyramidal
D. Trigonal bipyramidal, tetrahedral and square
Answer
559.2k+ views
Hint:With the valency of the central atom, find out the hybridization of the molecule. The hybridization of the molecule determines the shape of the molecule. We should also check for the presence of lone pairs and the change in shape due to that.
Complete step-by-step answer:${\rm I})$ $PC{l_4}^ + $ : In this molecule, the valency of central atom Phosphorus is 5 and due to the positive charge one electron is removed. So now the remaining 4 electrons bond with one Chlorine each.
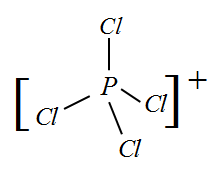
Since there are 4 bonds, the hybridization will be $s{p^3}$ and the shape corresponding to this hybridization is Tetrahedral.
${\rm I}{\rm I})$ $PC{l_4}^ - $ : The valency of Phosphorus is 5 and given a negative charge so now we will have 4 electrons bonded to Chlorine and one lone pair. Hence, the hybridization would be $s{p^3}d$ . Since we have a lone pair, the hybridization changes from $s{p^3}$ to $s{p^3}d$ .
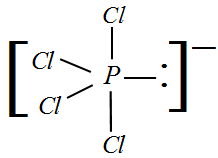
Therefore, for a molecule with a lone pair and $s{p^3}d$ hybridization, the shape would be see-saw.
${\rm I}{\rm I}{\rm I})$ $AsC{l_5}$ : Since Arsenic belongs to the same group as Phosphorus, we can write the valency of Arsenic to be 5. All the 5 electrons are bonded with one Chlorine each and hence we can deduce the hybridization to be $s{p^3}d$ and there are no lone pairs.
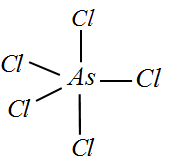
Therefore the shape would be trigonal bipyramidal.
Additional information: Hybridization of a molecule plays an important role in determining the shape of that molecule. Presence of a positive or negative charge or a lone pair changes the shape of the molecule.
Note:If the hybridization of the given molecule is $s{p^3}$ then it’s shape would be tetragonal. If the hybridization is $s{p^3}d$ without a lone pair then the shape would be trigonal bipyramidal and with a lone pair the shape would be see-saw.
Complete step-by-step answer:${\rm I})$ $PC{l_4}^ + $ : In this molecule, the valency of central atom Phosphorus is 5 and due to the positive charge one electron is removed. So now the remaining 4 electrons bond with one Chlorine each.
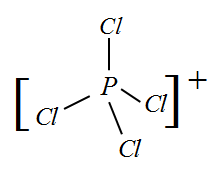
Since there are 4 bonds, the hybridization will be $s{p^3}$ and the shape corresponding to this hybridization is Tetrahedral.
${\rm I}{\rm I})$ $PC{l_4}^ - $ : The valency of Phosphorus is 5 and given a negative charge so now we will have 4 electrons bonded to Chlorine and one lone pair. Hence, the hybridization would be $s{p^3}d$ . Since we have a lone pair, the hybridization changes from $s{p^3}$ to $s{p^3}d$ .
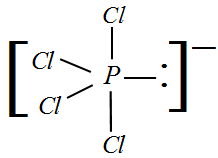
Therefore, for a molecule with a lone pair and $s{p^3}d$ hybridization, the shape would be see-saw.
${\rm I}{\rm I}{\rm I})$ $AsC{l_5}$ : Since Arsenic belongs to the same group as Phosphorus, we can write the valency of Arsenic to be 5. All the 5 electrons are bonded with one Chlorine each and hence we can deduce the hybridization to be $s{p^3}d$ and there are no lone pairs.

Therefore the shape would be trigonal bipyramidal.
Additional information: Hybridization of a molecule plays an important role in determining the shape of that molecule. Presence of a positive or negative charge or a lone pair changes the shape of the molecule.
Note:If the hybridization of the given molecule is $s{p^3}$ then it’s shape would be tetragonal. If the hybridization is $s{p^3}d$ without a lone pair then the shape would be trigonal bipyramidal and with a lone pair the shape would be see-saw.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




