
What are the products formed when $NaOH$ reacts with excess $S$ ?
(A) $N{a_2}{S_5},N{a_2}S{O_4}$
(B) $N{a_2}S,N{a_2}{S_2}{O_3}$
(C) $N{a_2}{S_5},N{a_2}{S_2}{O_3}$
(D) $N{a_2}{S_2}{O_3}$
Answer
565.5k+ views
Hint:We know that sodium hydroxide when boiled with an excess of sulfur, a disproportionate reaction occurs. In a disproportionate reaction, the same element in a compound is getting oxidized and reduced simultaneously. Here $S$ is undergoing a disproportionation reaction.
Complete answer:Sodium Hydroxide is an inorganic compound. It is also known as caustic soda. It is ionic and is a white crystalline solid. It has a formula $NaOH$ with cation $N{a^ + }$ and anion $O{H^ - }$ . Sulfur is a group $16$ element in the periodic table. It is non-metallic in nature and has a symbol $S$ . Sulfur is bright yellow, solid at room temperature. When sodium hydroxide is boiled with an excess of sulfur, the following reaction occurs:

It is a disproportionation reaction. A disproportionation reaction is a redox reaction in which the same element in a compound is getting oxidized and reduced simultaneously. So here sulfur $\left( S \right)$ is undergoing a disproportionation reaction. Its oxidation state changes from zero to $ - 2$ in sodium sulfide to $ + 4$ in sodium thiosulfate.
$N{a_2}S$ is sodium sulfide. It is a colorless solid and water-soluble. And it gives a strong alkaline solution when dissolved in water.
$N{a_2}{S_2}{O_3}$ is called sodium thiosulfate. It is an inorganic compound that is used as an antidote in cyanide poisoning and also an antifungal drug.
Thus, the correct option is $A$.
Note:It is important to write the correct stoichiometric coefficients in the equation. Different reactions occur if we change the stoichiometric coefficients. Look at the following equations:
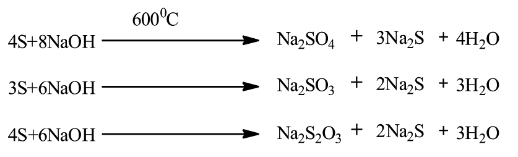
So if we compare these three equations, we can see that different products are formed if we change the stoichiometric coefficients.
Complete answer:Sodium Hydroxide is an inorganic compound. It is also known as caustic soda. It is ionic and is a white crystalline solid. It has a formula $NaOH$ with cation $N{a^ + }$ and anion $O{H^ - }$ . Sulfur is a group $16$ element in the periodic table. It is non-metallic in nature and has a symbol $S$ . Sulfur is bright yellow, solid at room temperature. When sodium hydroxide is boiled with an excess of sulfur, the following reaction occurs:

It is a disproportionation reaction. A disproportionation reaction is a redox reaction in which the same element in a compound is getting oxidized and reduced simultaneously. So here sulfur $\left( S \right)$ is undergoing a disproportionation reaction. Its oxidation state changes from zero to $ - 2$ in sodium sulfide to $ + 4$ in sodium thiosulfate.
$N{a_2}S$ is sodium sulfide. It is a colorless solid and water-soluble. And it gives a strong alkaline solution when dissolved in water.
$N{a_2}{S_2}{O_3}$ is called sodium thiosulfate. It is an inorganic compound that is used as an antidote in cyanide poisoning and also an antifungal drug.
Thus, the correct option is $A$.
Note:It is important to write the correct stoichiometric coefficients in the equation. Different reactions occur if we change the stoichiometric coefficients. Look at the following equations:

So if we compare these three equations, we can see that different products are formed if we change the stoichiometric coefficients.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




