
What are the postulates of Bohr’s model of an atom?
Answer
584.7k+ views
Hint: We must remember that in 1915, the Bohr model of the atom was given by Neil Bohr. It came into reality with the modification of Rutherford’s atomic model. Rutherford’s model suggested that a nucleus positively charged is surrounded by negatively charged electrons.
Bohr modified this atomic structure model by describing that electrons go in fixed orbitals called shells. He explained that each orbit contains a fixed energy level. Rutherford described the nucleus of an atom and Bohr modified that model into electrons and their energy levels.
Complete step by step answer:
We can give the postulates of Bohr’s model of an atom as follows,
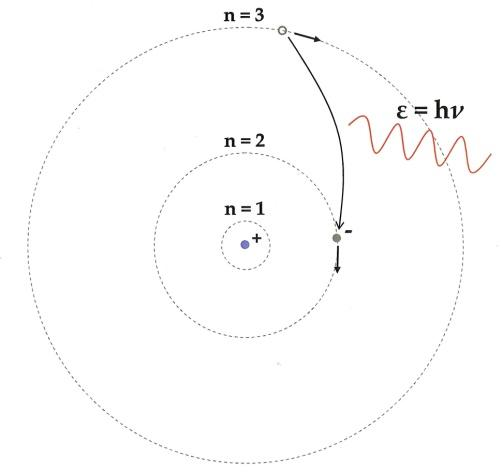
1.In an atom, electrons that are negatively charged spin around the positively charged nucleus in a specific circular path known as orbits or shells.
2.Each orbit or shell contains a fixed energy and the circular orbits are called orbital shells.
3.The energy levels are denoted by an integer (n=1, 2, 3…) called as the quantum number. This range of quantum numbers starts from the side of the nucleus with n=1 having the lowest energy level. The orbits n=1, 2, 3, 4… are labeled as K, L, M, N…. shells and when an electron reaches the lowest energy level, it is said to be in the ground state.
4.The electrons in an atom go from a lower energy level to a higher energy level by gaining the necessary energy and an electron goes from a higher energy level to lower energy level by losing energy.
Note:
Let us discuss on the limitations of Bohr’s atomic model:
1.Bohr’s model of an atom would not describe the Zeeman Effect (effect of magnetic field on the spectra of atoms).
2.Bohr’s model of an atom would not describe the Stark effect (effect of electric field on the spectra of atoms).
3.It disobeys the Heisenberg Uncertainty Principle.
4.It failed to explain about the spectra obtained from larger atoms and it does not predict the relative intensities of spectral lines.
5.The Bohr Model does not report for the fact that electromagnetic radiations are not emitted by accelerating electrons.
Bohr modified this atomic structure model by describing that electrons go in fixed orbitals called shells. He explained that each orbit contains a fixed energy level. Rutherford described the nucleus of an atom and Bohr modified that model into electrons and their energy levels.
Complete step by step answer:
We can give the postulates of Bohr’s model of an atom as follows,
1.In an atom, electrons that are negatively charged spin around the positively charged nucleus in a specific circular path known as orbits or shells.
2.Each orbit or shell contains a fixed energy and the circular orbits are called orbital shells.
3.The energy levels are denoted by an integer (n=1, 2, 3…) called as the quantum number. This range of quantum numbers starts from the side of the nucleus with n=1 having the lowest energy level. The orbits n=1, 2, 3, 4… are labeled as K, L, M, N…. shells and when an electron reaches the lowest energy level, it is said to be in the ground state.
4.The electrons in an atom go from a lower energy level to a higher energy level by gaining the necessary energy and an electron goes from a higher energy level to lower energy level by losing energy.

Note:
Let us discuss on the limitations of Bohr’s atomic model:
1.Bohr’s model of an atom would not describe the Zeeman Effect (effect of magnetic field on the spectra of atoms).
2.Bohr’s model of an atom would not describe the Stark effect (effect of electric field on the spectra of atoms).
3.It disobeys the Heisenberg Uncertainty Principle.
4.It failed to explain about the spectra obtained from larger atoms and it does not predict the relative intensities of spectral lines.
5.The Bohr Model does not report for the fact that electromagnetic radiations are not emitted by accelerating electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




