
How are the molecules arranged in the three states of matter?
Answer
558.3k+ views
Hint:The main three states of matter are solid, liquid, and gas. The matter is classified into these three states because of the nature of the arrangement of the particles and the intermolecular forces existing between the constituents of the matter.The strength of the intermolecular forces existing between the constituents of the matter decides the state of the matter.
Complete step-by-step answer:The three states the matters are solids, liquids, and gases.The diagrammatic representation of the solid particles is as follows:
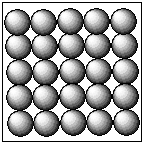
In the case of the solid, the constituent particles that are atoms, ions, molecules are held together by the strong intermolecular forces of the attraction. Hence, the particles of the solid substance are closely packed in a defined manner. This gives the shape of the particles to the solid substances.The diagrammatic representation of the liquid particles is as follows:
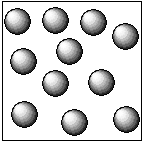
In the case of the liquid, the constituent particles that are atoms, ions, and molecules are held together by the intermolecular forces of the attraction which are weak compared to existing in the solid particles. Hence, the particles of the liquid substance are not closely packed. Therefore, the liquid does not possess a definite shape and volume. They occupy the shape of the container in which it is placed.The diagrammatic representation of the gas particles is as follows:
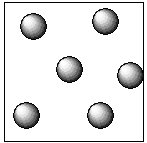
In the case of gases, the constituent particles that are atoms, ions, and molecules are held together by the weak intermolecular forces of the attraction which are weaker compared to existing in the liquid particles. Hence, the particles of the gaseous substance are not closely packed. Therefore, gases do not possess a definite shape and volume.
Note:The forces of the attraction existing between the particles of the same substance are known as intermolecular forces of the attraction.
The stronger the intermolecular forces more tightly will be the particles are arranged in the matter.
The different types of intermolecular forces include the different types of chemical bonds like hydrogen bond, covalent bond, ionic bond, metallic bond, the coordinate bond also weak forces like van der Waals forces, dipole-dipole interaction, London forces, etc. All these are leads to give specific shape and state to the matter.
Complete step-by-step answer:The three states the matters are solids, liquids, and gases.The diagrammatic representation of the solid particles is as follows:

In the case of the solid, the constituent particles that are atoms, ions, molecules are held together by the strong intermolecular forces of the attraction. Hence, the particles of the solid substance are closely packed in a defined manner. This gives the shape of the particles to the solid substances.The diagrammatic representation of the liquid particles is as follows:

In the case of the liquid, the constituent particles that are atoms, ions, and molecules are held together by the intermolecular forces of the attraction which are weak compared to existing in the solid particles. Hence, the particles of the liquid substance are not closely packed. Therefore, the liquid does not possess a definite shape and volume. They occupy the shape of the container in which it is placed.The diagrammatic representation of the gas particles is as follows:

In the case of gases, the constituent particles that are atoms, ions, and molecules are held together by the weak intermolecular forces of the attraction which are weaker compared to existing in the liquid particles. Hence, the particles of the gaseous substance are not closely packed. Therefore, gases do not possess a definite shape and volume.
Note:The forces of the attraction existing between the particles of the same substance are known as intermolecular forces of the attraction.
The stronger the intermolecular forces more tightly will be the particles are arranged in the matter.
The different types of intermolecular forces include the different types of chemical bonds like hydrogen bond, covalent bond, ionic bond, metallic bond, the coordinate bond also weak forces like van der Waals forces, dipole-dipole interaction, London forces, etc. All these are leads to give specific shape and state to the matter.
Recently Updated Pages
Master Class 7 English: Engaging Questions & Answers for Success

Master Class 7 Maths: Engaging Questions & Answers for Success

Master Class 7 Science: Engaging Questions & Answers for Success

Class 7 Question and Answer - Your Ultimate Solutions Guide

Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Trending doubts
The value of 6 more than 7 is A 1 B 1 C 13 D 13 class 7 maths CBSE

Convert 200 Million dollars in rupees class 7 maths CBSE

List of coprime numbers from 1 to 100 class 7 maths CBSE

AIM To prepare stained temporary mount of onion peel class 7 biology CBSE

The plural of Chief is Chieves A True B False class 7 english CBSE

Write a letter to the editor of the national daily class 7 english CBSE





