
What are the molecular formula, the structural formula, the condensed structural formula, and the abbreviated line formula for pentane?
Answer
540.3k+ views
Hint: In order to solve the question, we first need to look at the name of the compound then we will come to know from the suffix and prefix that exactly what the compound is. Pentane is an organic compound with 5 carbon atoms attached with single bonds.
Complete step-by-step answer:Pentane is a 5 carbon organic compound and since the suffix is ‘– ane’ we can say that there are only single bonds, i.e., it is a saturated molecule. There are no double or triple bonds.
The molecular formula of pentane is
$C_{5}H_{12}$
The structural formula of pentane is
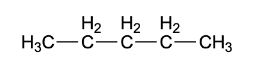
The chemical formula in which there is no presence of sigma or pi bonds is called condensed formula. The condensed structural formula of pentane is
$CH_{3}(CH_{2})_{3}CH_{3}$
There are two isomers for pentane-
Isopentane or 2-methylbutane – In this type of arrangement, 4 carbon atoms are arranged in a straight chain and the fifth carbon atom is attached to the second carbon of the straight chain to form a branch.
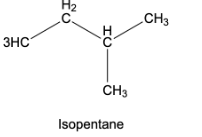
Neopentane or 2,2-dimethylpropane – Here, 3 carbon atoms are arranged one after the other to form a straight chain, while the other 2 carbon atoms are attached to the middle carbon atom of the straight chain to form 2 branches.
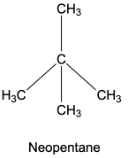
Note: The condensed formula gives the idea about the number of carbons, number of hydrogens, number of oxygen atoms, and the number of nitrogen atoms present in the compound very easily. By using bond line formulas, we can easily present the structures of the organic compounds very easily.
Complete step-by-step answer:Pentane is a 5 carbon organic compound and since the suffix is ‘– ane’ we can say that there are only single bonds, i.e., it is a saturated molecule. There are no double or triple bonds.
The molecular formula of pentane is
$C_{5}H_{12}$
The structural formula of pentane is
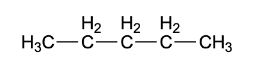
The chemical formula in which there is no presence of sigma or pi bonds is called condensed formula. The condensed structural formula of pentane is
$CH_{3}(CH_{2})_{3}CH_{3}$
There are two isomers for pentane-
Isopentane or 2-methylbutane – In this type of arrangement, 4 carbon atoms are arranged in a straight chain and the fifth carbon atom is attached to the second carbon of the straight chain to form a branch.

Neopentane or 2,2-dimethylpropane – Here, 3 carbon atoms are arranged one after the other to form a straight chain, while the other 2 carbon atoms are attached to the middle carbon atom of the straight chain to form 2 branches.

Note: The condensed formula gives the idea about the number of carbons, number of hydrogens, number of oxygen atoms, and the number of nitrogen atoms present in the compound very easily. By using bond line formulas, we can easily present the structures of the organic compounds very easily.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




