
Are the following names correct according to the IUPAC system of nomenclature? If not, give the correct names. 1-Methoxyethane-2-ol.
Answer
576.3k+ views
Hint: We know that Hydrocarbons are classified into alkane or or cycloalkanes, depending upon whether the carbon atoms of the molecule are arranged only in ring or in chain. Although these hydrocarbons have no functional groups, they constitute the framework on which functional groups are located in other classes of compounds, and provide an ideal starting point for studying and naming organic compounds.
Complete step by step answer:
According to the IUPAC rules of nomenclature for alkanes, there are certain rules that must be followed in order to determine the name of any alkane or unsaturated but substituted organic compound. These rules are:
(i) Determine the length of the longest parent carbon chain and name the longest continuous carbon chain which is being determined. (ii) Identify and name groups attached to this chain. (iii) Number the chain consecutively, starting at the end nearest a substituent group. (iv) Designate the location of each substituent group by an appropriate number and name. (v) Assemble the name, listing groups in alphabetical order using the full name (e.g. cyclopropyl before isobutyl). The prefixes di, tri, tetra etc., used to designate several groups of the same kind, are not considered when alphabetizing.
Based on the points mentioned above, the correct name of the organic compound should be 2-methoxy-ethan-1-ol and not 1-methoxyethan-2-ol. The structure of the organic compound 2-methoxy-ethan-1-ol is as follows:
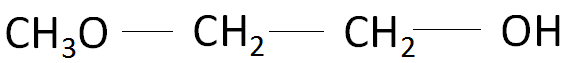
Note: In the IUPAC system, alcohols are named by changing the ending of the parent alkane name to\[ - ol\] . Alcohols are classified according to the number of carbon atoms attached to the carbon atom that is attached to the \[OH\] group.
Complete step by step answer:
According to the IUPAC rules of nomenclature for alkanes, there are certain rules that must be followed in order to determine the name of any alkane or unsaturated but substituted organic compound. These rules are:
(i) Determine the length of the longest parent carbon chain and name the longest continuous carbon chain which is being determined. (ii) Identify and name groups attached to this chain. (iii) Number the chain consecutively, starting at the end nearest a substituent group. (iv) Designate the location of each substituent group by an appropriate number and name. (v) Assemble the name, listing groups in alphabetical order using the full name (e.g. cyclopropyl before isobutyl). The prefixes di, tri, tetra etc., used to designate several groups of the same kind, are not considered when alphabetizing.
Based on the points mentioned above, the correct name of the organic compound should be 2-methoxy-ethan-1-ol and not 1-methoxyethan-2-ol. The structure of the organic compound 2-methoxy-ethan-1-ol is as follows:
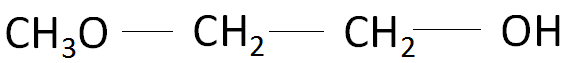
Note: In the IUPAC system, alcohols are named by changing the ending of the parent alkane name to\[ - ol\] . Alcohols are classified according to the number of carbon atoms attached to the carbon atom that is attached to the \[OH\] group.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




