
How are the following ethers prepared by Williamson synthesis?
A.Ethoxybenzene
B. 2-methoxy-2-methylpropane
Answer
574.2k+ views
Hint: In the Williamsons synthesis method, sodium alkoxide is made to react with an alkyl halide to give ethers as the major product. It is a nucleophilic substitution that undergoes with the ${{S}_{N}}2$ mechanism.
Complete Solution :
In order to answer our question, we need to learn about ethers and Williamson's synthesis. Ethers are classified as symmetrical or simple, based on if the aryl or alkyl group attached to the oxygen atom are the same, and mixed or unsymmetrical. If the two groups are different. The nomenclature of ethers are derived from the names of groups written as separate words in alphabetical order and adding the word ether at the end, be it aryl or alkyl group. According to IUPAC system of nomenclature, ethers are regarded as hydrocarbon derivatives in which a hydrogen atom is replaced by an -OR or -OAr groups. For the parent hydrocarbon, the larger (R) group is chosen. In ethers, the two bond pairs and two lone pairs of electrons on oxygen are arranged approximately in a tetrahedral arrangement The bond angle is slightly greater than the tetrahedral angle due to (bp-bp-repulsion) the repulsive interaction between the two bulky (-R) groups. The (C- O) bond length is almost the same as in alcohols.
Williamsons synthesis is an important method to prepare all types of ether. Alkyl halide is allowed to react with sodium alkoxide.
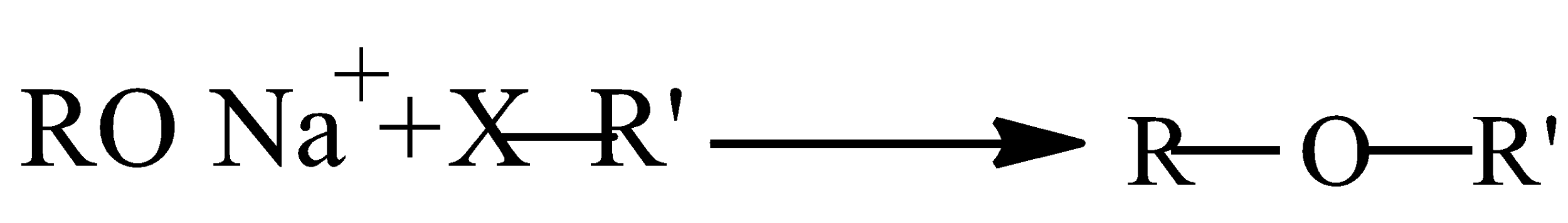
Now, we can write the preparation reactions of A. Ethoxybenzene and B. 2-methoxy-2-methylpropane as:
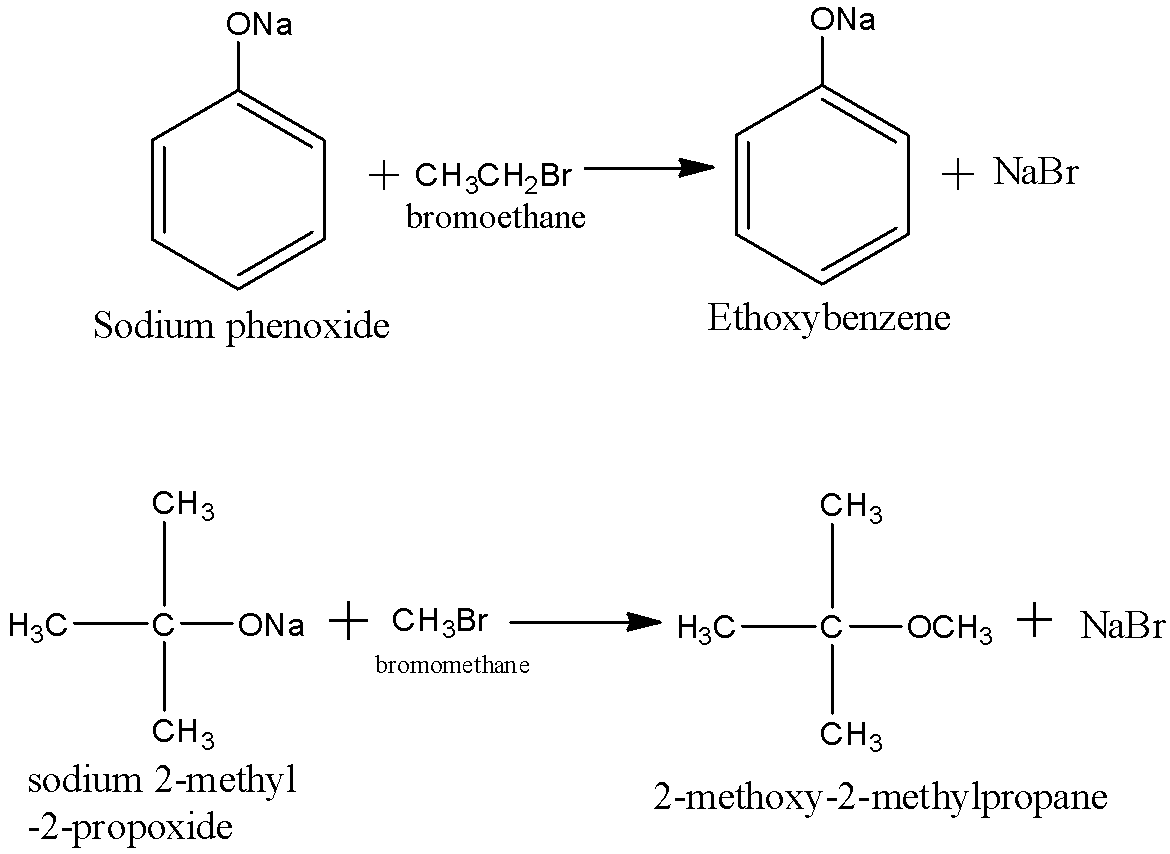
So our answers would be Ethoxybenzene and 2 methoxy 2 methylpropane respectively.
Note: It is to be noted that along with the major product as either, a side product NaBr is also formed. Moreover, best results are obtained if the alkyl halide is primary. Phenols can be also converted to ethers by this method.
Complete Solution :
In order to answer our question, we need to learn about ethers and Williamson's synthesis. Ethers are classified as symmetrical or simple, based on if the aryl or alkyl group attached to the oxygen atom are the same, and mixed or unsymmetrical. If the two groups are different. The nomenclature of ethers are derived from the names of groups written as separate words in alphabetical order and adding the word ether at the end, be it aryl or alkyl group. According to IUPAC system of nomenclature, ethers are regarded as hydrocarbon derivatives in which a hydrogen atom is replaced by an -OR or -OAr groups. For the parent hydrocarbon, the larger (R) group is chosen. In ethers, the two bond pairs and two lone pairs of electrons on oxygen are arranged approximately in a tetrahedral arrangement The bond angle is slightly greater than the tetrahedral angle due to (bp-bp-repulsion) the repulsive interaction between the two bulky (-R) groups. The (C- O) bond length is almost the same as in alcohols.
Williamsons synthesis is an important method to prepare all types of ether. Alkyl halide is allowed to react with sodium alkoxide.
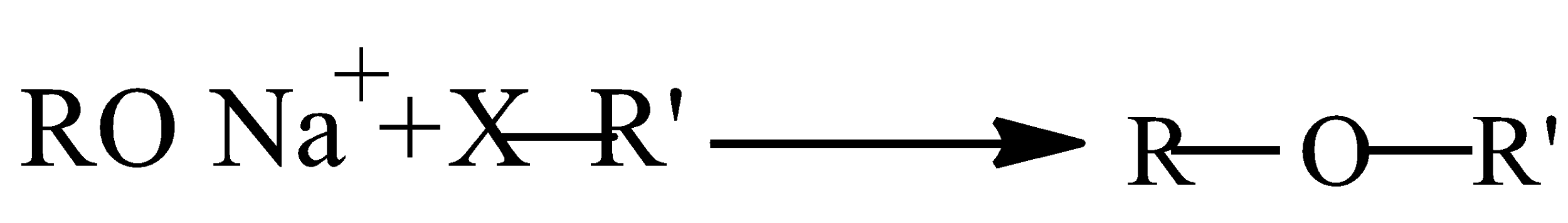
Now, we can write the preparation reactions of A. Ethoxybenzene and B. 2-methoxy-2-methylpropane as:

So our answers would be Ethoxybenzene and 2 methoxy 2 methylpropane respectively.
Note: It is to be noted that along with the major product as either, a side product NaBr is also formed. Moreover, best results are obtained if the alkyl halide is primary. Phenols can be also converted to ethers by this method.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

Draw a labelled sketch of the human eye class 12 physics CBSE

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




