
What are strong and weak acids? In the following list of acids, separate strong acids from weak acids. Hydrochloric acid, citric acid, acetic acid, nitric acid, formic acid, sulphuric acid.
Answer
533.1k+ views
Hint:We know that there are two types of substance, i.e., acids and base, these are classified on the basis of ions produced by their solution, i.e., hydrogen ions and hydroxyl ions. Extending the ions produced tells whether the compound is strong or weak.
Complete step-by-step answer:We know that there are two types of substance, i.e., acids and base, these are classified on the basis of ions produced by their solution. The acids produce hydrogen ions whereas the base produces hydroxyl ions.
We can decide the compound is a strong acid or weak acid on the basis of the extent of hydrogen ions produced in the solution, so the acids that get fully ionized in the solution then it is a strong acid and the acids get partially ionized in the solution then it is a weak acid.
Hydrochloric acid having the formula HCl is a strong acid because when it is dissolved in water it completely dissociates into ${{H}^{+}}$ and $C{{l}^{-}}$ ions.
Citric acid having formula ${{C}_{6}}{{H}_{8}}{{O}_{7}}$ is a weak acid because when it is dissolved in water it partially dissociates into ions and remains in equilibrium. The structure is given below:
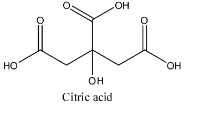
Acetic acid having formula $C{{H}_{3}}COOH$ is a weak acid because when it is dissolved in water it partially dissociates into ions and remains in equilibrium.
Nitric acid having formula $HN{{O}_{3}}$ is a strong acid because when it is dissolved in water it completely dissociates into ${{H}^{+}}$ and $NO_{3}^{-}$ ions.
Formic acid having the formula HCOOH is a weak acid because when it is dissolved in water it partially dissociates into ions and remains in equilibrium.
Sulphuric acid having formula ${{H}_{2}}S{{O}_{4}}$ is a strong acid because when it is dissolved in water it completely dissociates into ${{H}^{+}}$ and $SO_{4}^{2-}$ ions.
Note: In the same way we can classify the bases as a strong base and weak base, compounds that ionize fully are called strong bases, and compounds that partially ionize are called weak bases.
Complete step-by-step answer:We know that there are two types of substance, i.e., acids and base, these are classified on the basis of ions produced by their solution. The acids produce hydrogen ions whereas the base produces hydroxyl ions.
We can decide the compound is a strong acid or weak acid on the basis of the extent of hydrogen ions produced in the solution, so the acids that get fully ionized in the solution then it is a strong acid and the acids get partially ionized in the solution then it is a weak acid.
Hydrochloric acid having the formula HCl is a strong acid because when it is dissolved in water it completely dissociates into ${{H}^{+}}$ and $C{{l}^{-}}$ ions.
Citric acid having formula ${{C}_{6}}{{H}_{8}}{{O}_{7}}$ is a weak acid because when it is dissolved in water it partially dissociates into ions and remains in equilibrium. The structure is given below:

Acetic acid having formula $C{{H}_{3}}COOH$ is a weak acid because when it is dissolved in water it partially dissociates into ions and remains in equilibrium.
Nitric acid having formula $HN{{O}_{3}}$ is a strong acid because when it is dissolved in water it completely dissociates into ${{H}^{+}}$ and $NO_{3}^{-}$ ions.
Formic acid having the formula HCOOH is a weak acid because when it is dissolved in water it partially dissociates into ions and remains in equilibrium.
Sulphuric acid having formula ${{H}_{2}}S{{O}_{4}}$ is a strong acid because when it is dissolved in water it completely dissociates into ${{H}^{+}}$ and $SO_{4}^{2-}$ ions.
Note: In the same way we can classify the bases as a strong base and weak base, compounds that ionize fully are called strong bases, and compounds that partially ionize are called weak bases.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




