
What are some examples of valence electrons?
Answer
532.2k+ views
Hint: Valence electrons are the electrons in the outermost shell. Valence electrons are the electrons in the outer energy level of an atom .They participate in interactions with other atoms. Valence electrons are generally the electrons that are farthest from the nucleus and as a result, they may be attracted as much or more by the nucleus of another atom than they are by their own nucleus.
Complete step by step answer:
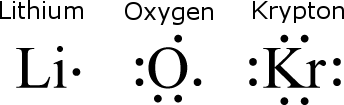
Because valence electrons are so important, atoms are often represented by simple diagrams that show only their valence electrons.
These are called electron dot diagrams, and three are shown below.
In this type of diagram, an element's chemical symbol is surrounded by dots that represent the valence electrons.
Typically, the dots are drawn as if there is a square surrounding the element symbol with up to two dots per side.
An element never has more than eight valence electrons, so there can’t be more than eight dots per atom.
Note: Valence electrons are the s and p electrons in the outermost shell.
The electrons present in the inner shell are core electrons.
When we study and observe the atom of an element, we come across tiny subatomic particles called valence electrons.
Lewis structures help us to track the valence electrons and predict the types of bond.
Valence electrons are all arranged in different orbitals or shells and are mostly negatively charged particles.
These electrons are responsible for interaction between atoms and the formation of chemical bonds.
However, not all electrons are associated with the atom. Only the electrons present in the outermost shell can participate in the formation of a chemical bond or a molecule. Such types of electrons are called valence electrons.
Complete step by step answer:
Because valence electrons are so important, atoms are often represented by simple diagrams that show only their valence electrons.
These are called electron dot diagrams, and three are shown below.
In this type of diagram, an element's chemical symbol is surrounded by dots that represent the valence electrons.
Typically, the dots are drawn as if there is a square surrounding the element symbol with up to two dots per side.
An element never has more than eight valence electrons, so there can’t be more than eight dots per atom.

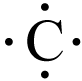
Note: Valence electrons are the s and p electrons in the outermost shell.
The electrons present in the inner shell are core electrons.
When we study and observe the atom of an element, we come across tiny subatomic particles called valence electrons.
Lewis structures help us to track the valence electrons and predict the types of bond.
Valence electrons are all arranged in different orbitals or shells and are mostly negatively charged particles.
These electrons are responsible for interaction between atoms and the formation of chemical bonds.
However, not all electrons are associated with the atom. Only the electrons present in the outermost shell can participate in the formation of a chemical bond or a molecule. Such types of electrons are called valence electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




