
What are some examples of electronic configuration?
Answer
521.4k+ views
Hint: The distribution of electrons in an element's atomic orbitals is defined by its electron configuration. Atomic electron configurations follow a standard notation in which all electron-containing atomic subshells are arranged in a series (with the number of electrons they possess written in superscript).
Complete step by step answer:
Electronic configuration of any atom is done on the basis of the number of electrons present in it. The principal quantum number determines the maximum number of electrons that can be accommodated in a shell (n). The shell number is expressed by the formula $ 2{n^2} $ , where n is the number of shells.
The azimuthal quantum number (abbreviated as ‘l') determines the subshells into which electrons are spread. The value of the principal quantum number, n, determines the value of this quantum number. As a result, when $ n = 4 $ , four separate subshells are possible. When $ n = 4 $ is used. The s, p, d, and f subshells are named after the $ l = 0 $ , $ l = 1 $ , $ l = 2 $ , and $ l = 3 $ subshells, respectively. The formula $ 2*(2l + 1) $ gives the maximum number of electrons that can be accommodated by a subshell. As a result, the s, p, d, and f subshells can each hold a maximum of $ 2 $ , $ 6 $ , $ 10 $ , or $ 14 $ electrons.
The electrons are filled in the subshell under Aufbau’s Principle which states that lower orbitals will be filled first. The order in which the electrons are filled in the subshells are listed below -
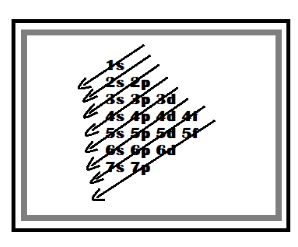
Let’s take some examples and see their electronic configuration –
Helium: Since the atomic number of helium is $ 2 $ , it has $ 2 $ electrons in its ground state. According to Aufbau principle, firstly $ 1s $ will be filled.
The electronic configuration of Helium is discussed below –
$ 1{s^2} $
Chlorine: Since, the atomic number of Chlorine is $ 17 $ , it has $ 17 $ electrons in its ground state. Firstly $ 2 $ electrons will be filled in $ 1s $ subshell, then $ 2 $ electrons will be filled in $ 2s $ subshell, then $ 6 $ electrons will be filled in $ 2p $ subshell and it continues.
The electronic configuration of Chlorine is discussed below –
$ 1{s^2},2{s^2},2{p^6},3{s^2},3{p^5} $
Tantalum: Since the atomic number of Tantalum is $ 73 $ , it has $ 73 $ electrons in its ground state. The electronic configuration of Tantalum is discussed below –
$ 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^{10}}4{p^6}5{s^2}4{d^{10}}5{p^6}6{s^2}4{f^{14}}5{d^3} $
Note:
Electron configurations can be used to determine an element's valency, predict the properties of a group of elements (elements with identical electron configurations appear to exhibit similar properties), and interpret atomic spectra.
Complete step by step answer:
Electronic configuration of any atom is done on the basis of the number of electrons present in it. The principal quantum number determines the maximum number of electrons that can be accommodated in a shell (n). The shell number is expressed by the formula $ 2{n^2} $ , where n is the number of shells.
The azimuthal quantum number (abbreviated as ‘l') determines the subshells into which electrons are spread. The value of the principal quantum number, n, determines the value of this quantum number. As a result, when $ n = 4 $ , four separate subshells are possible. When $ n = 4 $ is used. The s, p, d, and f subshells are named after the $ l = 0 $ , $ l = 1 $ , $ l = 2 $ , and $ l = 3 $ subshells, respectively. The formula $ 2*(2l + 1) $ gives the maximum number of electrons that can be accommodated by a subshell. As a result, the s, p, d, and f subshells can each hold a maximum of $ 2 $ , $ 6 $ , $ 10 $ , or $ 14 $ electrons.
The electrons are filled in the subshell under Aufbau’s Principle which states that lower orbitals will be filled first. The order in which the electrons are filled in the subshells are listed below -

Let’s take some examples and see their electronic configuration –
Helium: Since the atomic number of helium is $ 2 $ , it has $ 2 $ electrons in its ground state. According to Aufbau principle, firstly $ 1s $ will be filled.
The electronic configuration of Helium is discussed below –
$ 1{s^2} $
Chlorine: Since, the atomic number of Chlorine is $ 17 $ , it has $ 17 $ electrons in its ground state. Firstly $ 2 $ electrons will be filled in $ 1s $ subshell, then $ 2 $ electrons will be filled in $ 2s $ subshell, then $ 6 $ electrons will be filled in $ 2p $ subshell and it continues.
The electronic configuration of Chlorine is discussed below –
$ 1{s^2},2{s^2},2{p^6},3{s^2},3{p^5} $
Tantalum: Since the atomic number of Tantalum is $ 73 $ , it has $ 73 $ electrons in its ground state. The electronic configuration of Tantalum is discussed below –
$ 1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}3{d^{10}}4{p^6}5{s^2}4{d^{10}}5{p^6}6{s^2}4{f^{14}}5{d^3} $
Note:
Electron configurations can be used to determine an element's valency, predict the properties of a group of elements (elements with identical electron configurations appear to exhibit similar properties), and interpret atomic spectra.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




