
What are mesomers? Can we apply CIP nomenclature to mesomers to make R and S configurations?
Answer
492.9k+ views
Hint: Any compound with two chiral centers can be called mesomers. They are also called meso-compounds. Their net rotation is zero. CIP system is used to assign R and S configurations to the compounds. Take an example of a mesomer, and assign R and S to the two chiral centers.
Complete answer:
Mesomers are the type of organic compounds in which two chiral carbons are present and these two chiral carbons are similar. In these two compounds, the net rotation of the plane-polarized light is zero. They are also called meso-compounds.
For example- 2,3-Dichlorobutane,
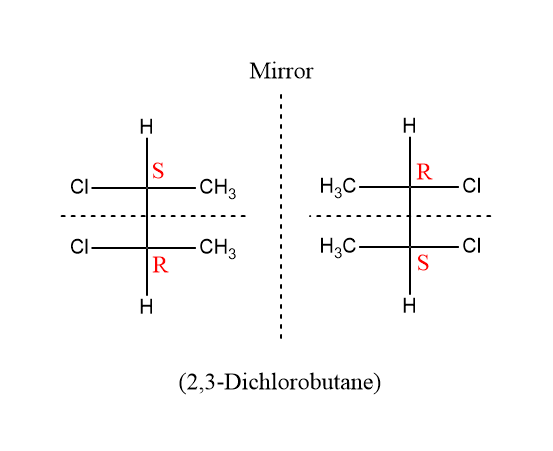
In this image it can be seen that the two mirror images are superimposable to each other and also each of them can be divided into two mirror-image halves by the horizontal line.
The full form of CIP nomenclature is Cahn-Ingold-Prelog system of nomenclature. It is a set of rules that allows to unambiguously define the stereochemical configuration of any stereocenter, using the designations 'R ' or ‘S’. The designation ‘R’ stands for Right-handed and ‘S’ stands for left-handed.
The CIP system can be used to name mesomers. So according to CIP system of nomenclature the name of left handed structure is \[(2S,3R) - 2,3 - \]Dichlorobutane and the name of the right handed structure is $\left( {2R,3S} \right) - 2,3 - $Dichlorobutane.
Note:
The main criterion to identify a meso compound is that it should have two or more than two stereocenters. And the stereochemistry should be R and S. Also one internal plane should divide the above and the lower plane into equal halves. In Meso compounds the net rotation is zero as the two images cancel out each other’s rotation resulting in zero net rotation.
Complete answer:
Mesomers are the type of organic compounds in which two chiral carbons are present and these two chiral carbons are similar. In these two compounds, the net rotation of the plane-polarized light is zero. They are also called meso-compounds.
For example- 2,3-Dichlorobutane,

In this image it can be seen that the two mirror images are superimposable to each other and also each of them can be divided into two mirror-image halves by the horizontal line.
The full form of CIP nomenclature is Cahn-Ingold-Prelog system of nomenclature. It is a set of rules that allows to unambiguously define the stereochemical configuration of any stereocenter, using the designations 'R ' or ‘S’. The designation ‘R’ stands for Right-handed and ‘S’ stands for left-handed.
The CIP system can be used to name mesomers. So according to CIP system of nomenclature the name of left handed structure is \[(2S,3R) - 2,3 - \]Dichlorobutane and the name of the right handed structure is $\left( {2R,3S} \right) - 2,3 - $Dichlorobutane.
Note:
The main criterion to identify a meso compound is that it should have two or more than two stereocenters. And the stereochemistry should be R and S. Also one internal plane should divide the above and the lower plane into equal halves. In Meso compounds the net rotation is zero as the two images cancel out each other’s rotation resulting in zero net rotation.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




