
What are good conductors of heat?
Answer
510.6k+ views
Hint: An object or type of material which allows the flow of charge or electric current in more than one direction is known as a conductor whereas the objects which do not allow the flow of electrons are known as insulators.
Complete answer:
We can explain the difference between the conduction of materials on the basis of electron band theory as follows:
Conductors: In these solids, there is no band gap between the valence band and conduction band that means the electrons are free to move between valence band and conduction band and thus allow the flow of electric current through it. Good conducting materials generally include metals, electrolytes, superconductors, semiconductors and some non-metallic elements like graphite and conductive polymers.
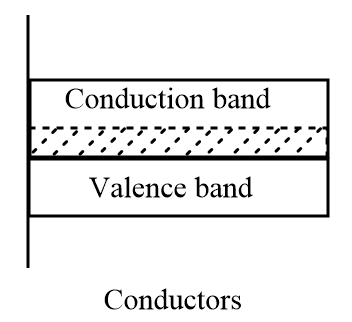
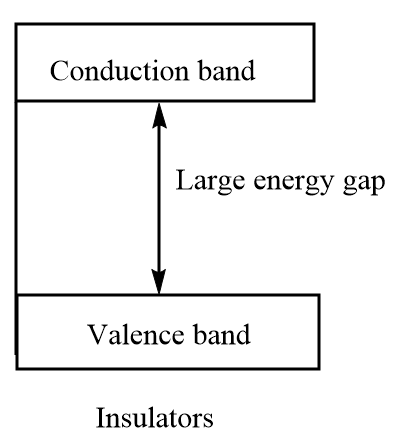
Insulators: In these solids, there is a large band gap between the valence band and the conduction band. The valence band is full of electrons but the movement of electrons from valence band to conduction band is prohibited. Therefore, these solids do not conduct electricity. Some of the most common examples of insulators are wood, plastic, glass, etc.
Hence, we can conclude that the metals are the good conductors of heat.
Note:
Remember that the shielding effect on an atom is defined as a reduction in the effective nuclear charge on the electron cloud because of the different attractive forces on the electron in an atom. Effective nuclear charge can be represented in terms of screening constant as ${Z_{eff}} = Z - \sigma $.
Complete answer:
We can explain the difference between the conduction of materials on the basis of electron band theory as follows:
Conductors: In these solids, there is no band gap between the valence band and conduction band that means the electrons are free to move between valence band and conduction band and thus allow the flow of electric current through it. Good conducting materials generally include metals, electrolytes, superconductors, semiconductors and some non-metallic elements like graphite and conductive polymers.

Insulators: In these solids, there is a large band gap between the valence band and the conduction band. The valence band is full of electrons but the movement of electrons from valence band to conduction band is prohibited. Therefore, these solids do not conduct electricity. Some of the most common examples of insulators are wood, plastic, glass, etc.

Hence, we can conclude that the metals are the good conductors of heat.
Note:
Remember that the shielding effect on an atom is defined as a reduction in the effective nuclear charge on the electron cloud because of the different attractive forces on the electron in an atom. Effective nuclear charge can be represented in terms of screening constant as ${Z_{eff}} = Z - \sigma $.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




