
How are following conversions carried out?
Propene to Propan-2-ol
Answer
530.7k+ views
Hint:The conversion Propene to Propan-2-ol follows Markovnikov's rule.
Propene in presence of water and acid produces propan-2-ol.
Complete step by step answer:
We can convert Propene to Propan-2-ol by reaction of the propene with acid.
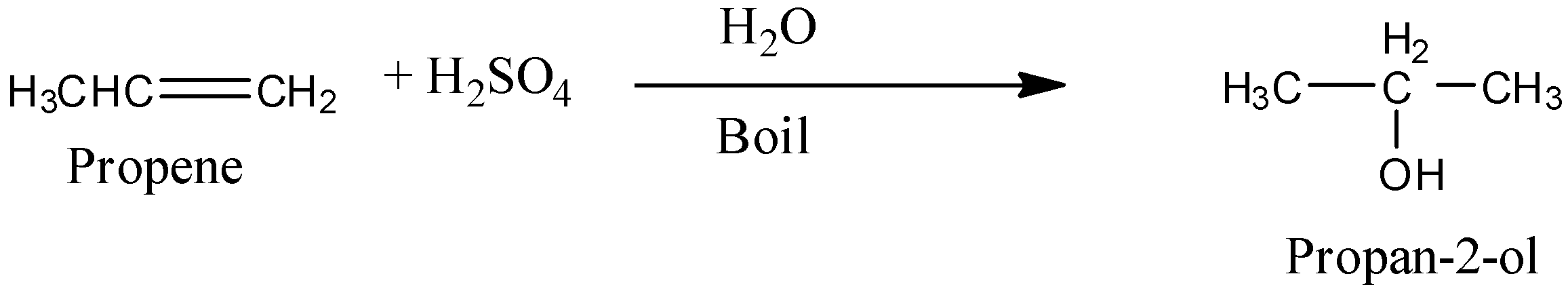
Propene reacts with acid in presence of water to produce Propan-2-ol.
We can write the mechanism of this reaction in the following way:
Step 1:
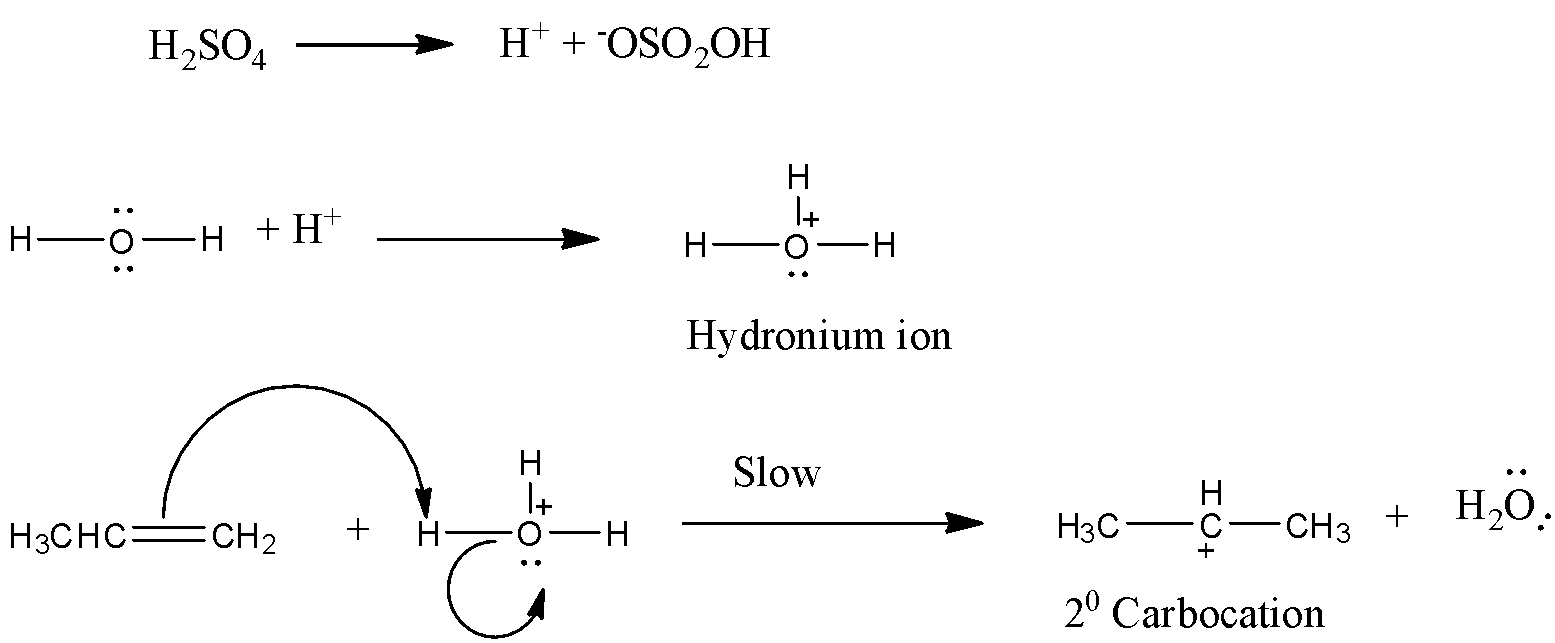
The propene reacts with an hydronium ion to generate a 2◦ carbocation
Step 2:
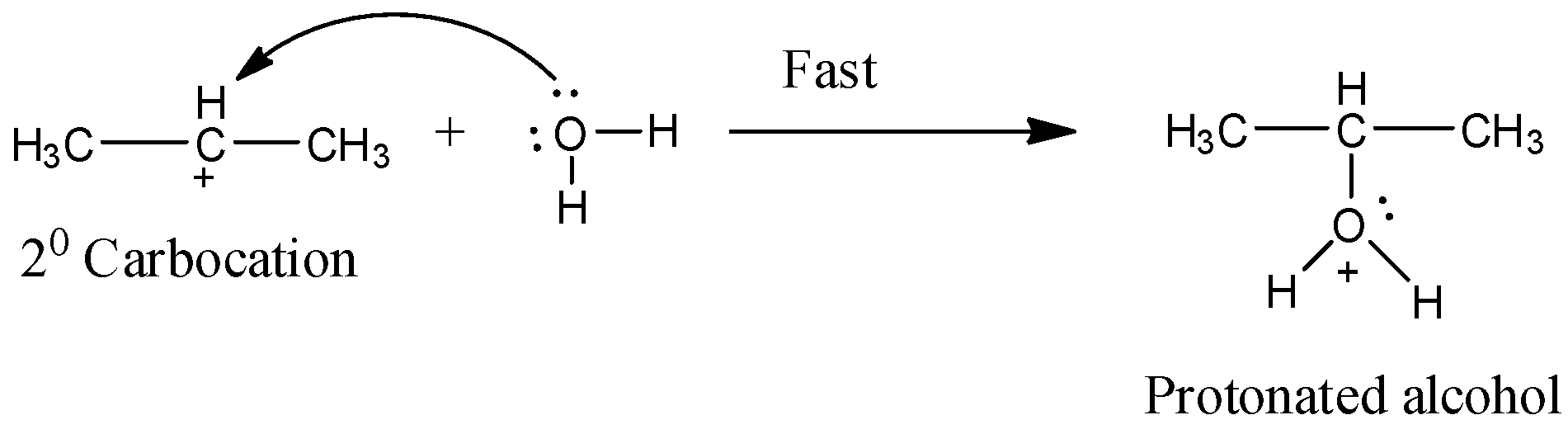
In this step, the 2◦ carbocation reacts with the water mole generating a protonated alcohol
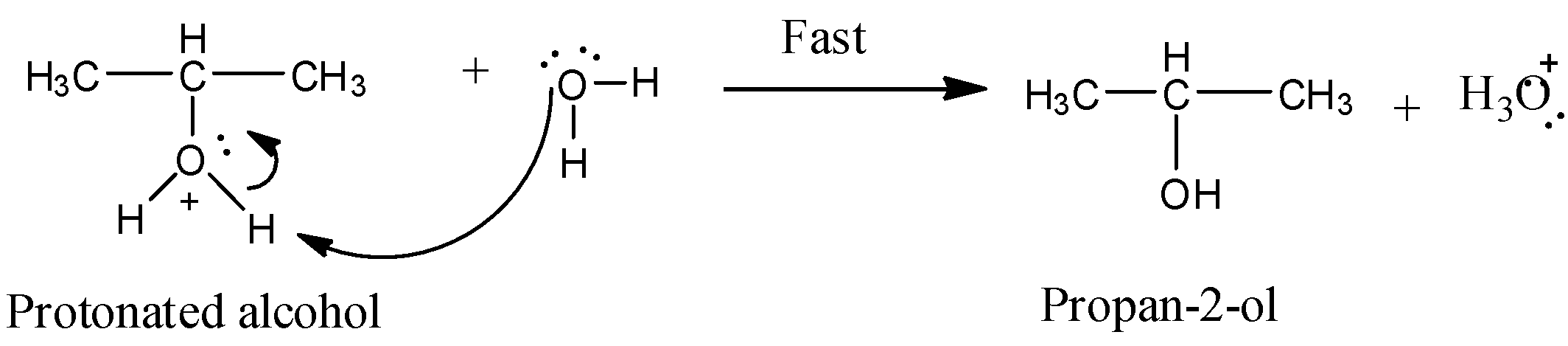
Step 3:
In this step protonated alcohol reacts with the water molecule generating the product Propan-2-ol.
Additional information:
Markovnikov’s rule is applicable in the addition reaction. According to this rule, the hydrogen atom gets attached to the more hydrogen substituted carbon atom and the halide atom gets attached to the carbon having more alkyl groups, that is, to the carbon having lesser hydrogen atoms. Markovnikov’s rule is also marked by the presence of a carbocation.
Propene has a low density, it is resistant to heat, and are chemically inert, it has stem barrier property which is used for the protection of food, it has good transparency and is also stretchable, therefore, it is used in the fibre and film. Propene also undergoes additional polymerization to generate polypropene, which is a commercially available thermoplastic.
Note:
The Markovnikov's rule is marked by the presence of a carbocation. According to this rule, the hydrogen atom gets attached to the more substituted carbon atom, while the halide atom gets attached to the less substituted carbon atom.
Propene in presence of water and acid produces propan-2-ol.
Complete step by step answer:
We can convert Propene to Propan-2-ol by reaction of the propene with acid.
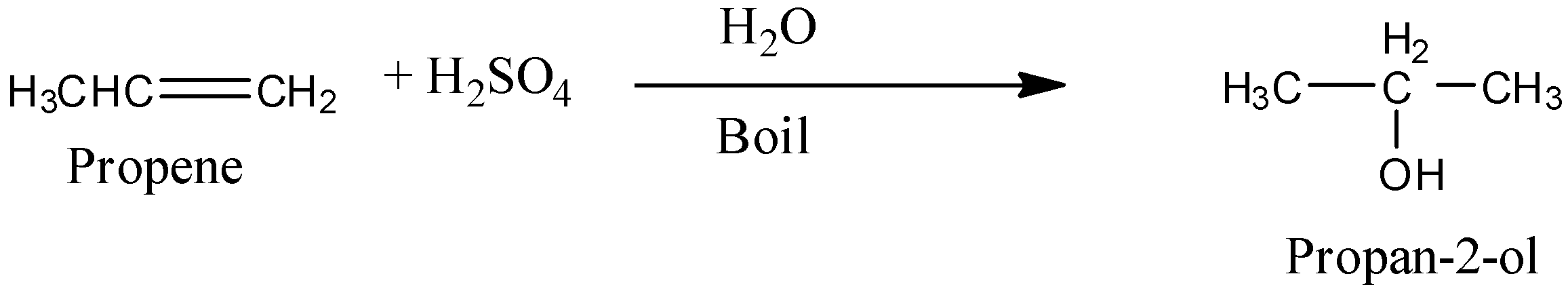
Propene reacts with acid in presence of water to produce Propan-2-ol.
We can write the mechanism of this reaction in the following way:
Step 1:

The propene reacts with an hydronium ion to generate a 2◦ carbocation
Step 2:

In this step, the 2◦ carbocation reacts with the water mole generating a protonated alcohol
Step 3:
In this step protonated alcohol reacts with the water molecule generating the product Propan-2-ol.

Additional information:
Markovnikov’s rule is applicable in the addition reaction. According to this rule, the hydrogen atom gets attached to the more hydrogen substituted carbon atom and the halide atom gets attached to the carbon having more alkyl groups, that is, to the carbon having lesser hydrogen atoms. Markovnikov’s rule is also marked by the presence of a carbocation.
Propene has a low density, it is resistant to heat, and are chemically inert, it has stem barrier property which is used for the protection of food, it has good transparency and is also stretchable, therefore, it is used in the fibre and film. Propene also undergoes additional polymerization to generate polypropene, which is a commercially available thermoplastic.
Note:
The Markovnikov's rule is marked by the presence of a carbocation. According to this rule, the hydrogen atom gets attached to the more substituted carbon atom, while the halide atom gets attached to the less substituted carbon atom.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




