
What are examples of geometric isomers?
Answer
528.6k+ views
Hint: Isomers are the compounds having the same molecular formula but they differ in structural formulas. There are various types of isomers like, position isomers, functional isomers, geometrical isomers, stereoisomers, etc. They all only differ in arrangement of the atoms in the structures.
Complete answer:
Isomerism is the property of any compound to have the same molecular formula but different structural formulas. The compounds having hindered rotation in space around a specific carbon – carbon double bond or single bond, that is the alteration in the spatial arrangement of molecules around the carbon atoms result in isomerism called geometrical isomerism and the isomers are called geometric isomers. There are 2 types of geometric isomers, ‘cis’ and ‘trans’.
-cis isomers: when similar groups are present on the same side of the double bonds, then they are termed as cis.
- trans isomers: when similar groups are present on the opposite sides of the double bonds then they are called trans isomers.
Some examples of cis and trans isomers are as follows:
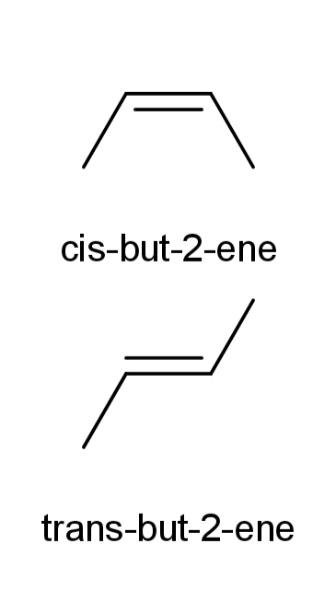
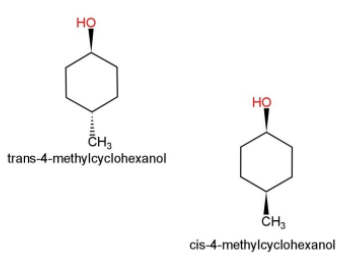
Cis, shows the alkyl groups or the functional groups on the same side of the carbon atom, while trans shows the alkyl and functional groups in both the cases on different sides of the carbon atoms.
Hence, cis and trans isomers are examples of geometric isomers.
Note:
These cis and trans isomers have same chemical formula but they differ in their physical properties. Trans isomers have high melting points due to symmetry than cis isomers. While, cis isomers have high boiling point than trans, due to the presence of polarity due to dipole moment.
Complete answer:
Isomerism is the property of any compound to have the same molecular formula but different structural formulas. The compounds having hindered rotation in space around a specific carbon – carbon double bond or single bond, that is the alteration in the spatial arrangement of molecules around the carbon atoms result in isomerism called geometrical isomerism and the isomers are called geometric isomers. There are 2 types of geometric isomers, ‘cis’ and ‘trans’.
-cis isomers: when similar groups are present on the same side of the double bonds, then they are termed as cis.
- trans isomers: when similar groups are present on the opposite sides of the double bonds then they are called trans isomers.
Some examples of cis and trans isomers are as follows:


Cis, shows the alkyl groups or the functional groups on the same side of the carbon atom, while trans shows the alkyl and functional groups in both the cases on different sides of the carbon atoms.
Hence, cis and trans isomers are examples of geometric isomers.
Note:
These cis and trans isomers have same chemical formula but they differ in their physical properties. Trans isomers have high melting points due to symmetry than cis isomers. While, cis isomers have high boiling point than trans, due to the presence of polarity due to dipole moment.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




