
How are Buna-s and nylon-6, 6 prepared? Give their uses.
Answer
589.2k+ views
Hint: Buna-s and nylon-6,6 are the synthetic polymers. They can be prepared by the polymerization reaction of their respective monomers. Polymer is a chain with more than 1000 monomers in its structure. The preparation of a polymer from monomers is called polymerization.
Complete step by step answer:
In the question it is given that to prepare Buna-s and nylon-6,6 polymers.
-First we should know the monomeric units to prepare Buna-s and nylon-6,6.
Buna-S
-The monomers used to prepare Buna-S polymer are 1,3-butadiene and styrene.
-The structures of 1,3-butadiene and styrene are as follows.
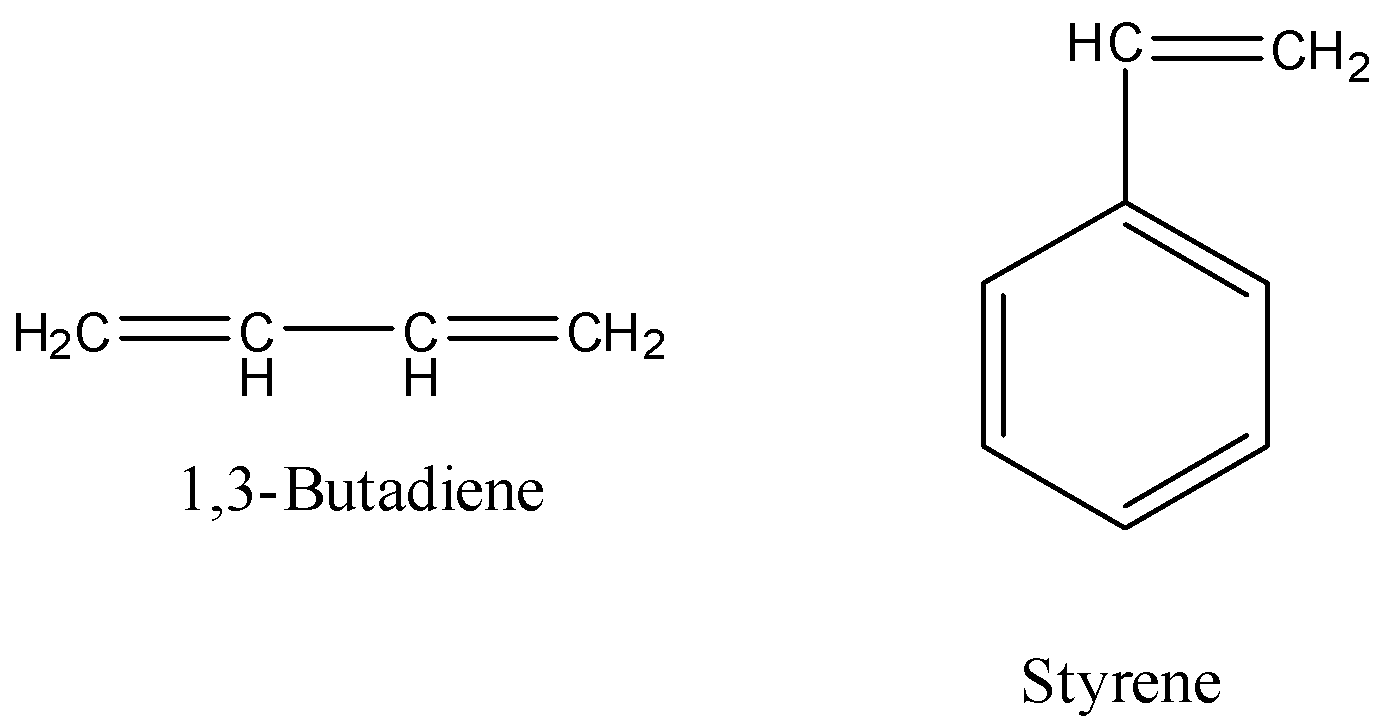
-The preparation of Buna-S is as follows.
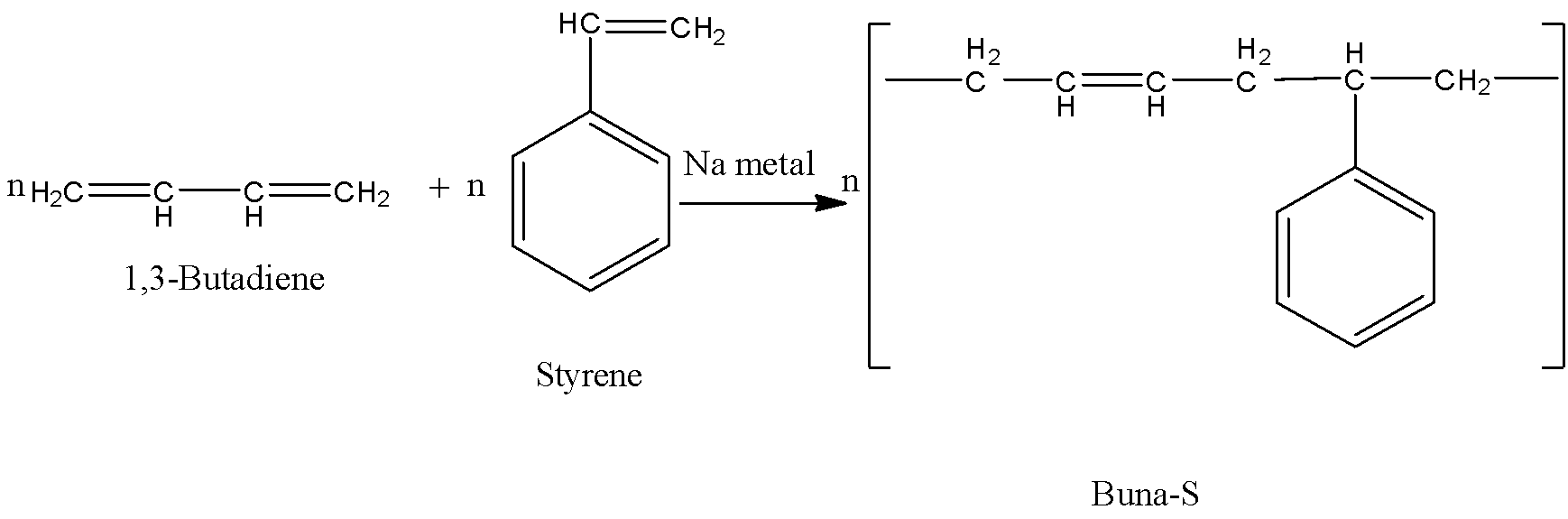
-In the above reaction 1,3-butadiene reacts with styrene in presence of sodium metal and forms Buna-S.
Uses of Buna-S:
-Buna-S is widely used in coated papers.
-It is used as building applications like as a sealing and binding agent
-Moreover it is used in rubber cutting boards.
-The monomers used to prepare Nylon-6,6 are adipic acid and hexamethylenediamine.
-The structures of adipic acid and hexamethylenediamine are as follows.
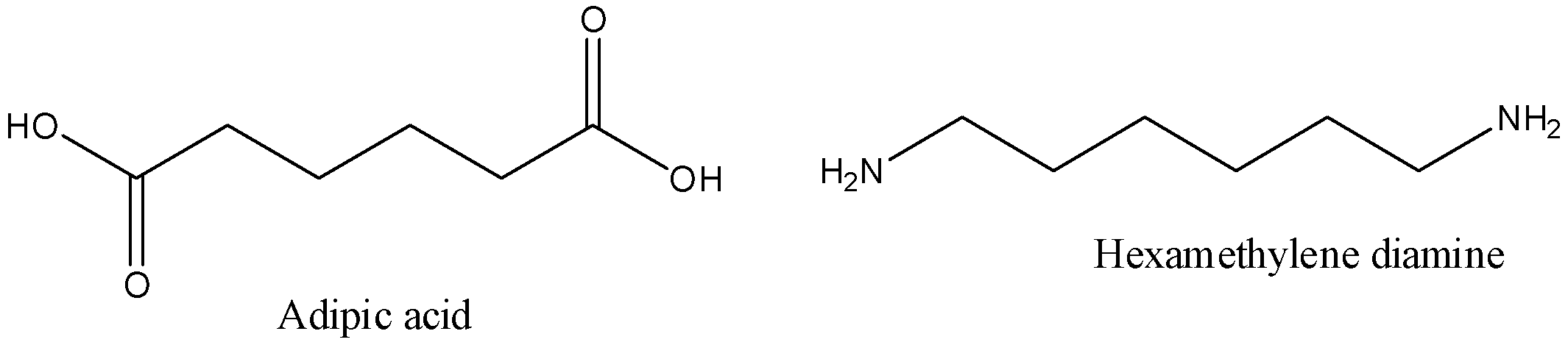
-The preparation of Nylon-6,6 is as follows.

-Nylon-6,6 is prepared by condensation polymerization of hexamethylene diamine with adipic acid at high temperature and pressure.
Uses of Nylon-6,6:
It is used in fabrication sheets, bristles for brushes.
Nylon-6,6 is waterproof in nature, so it is used to make swimwear.
Note: Buna-s is prepared through addition polymerization reactions of 1,3-butadiene and styrene. Nylon-6,6 is prepared by the condensation polymerization of adipic acid and hexamethylenediamine. In addition reaction there is no formation of side products but in condensation polymerization there is a formation of side products like methanol and water.
Complete step by step answer:
In the question it is given that to prepare Buna-s and nylon-6,6 polymers.
-First we should know the monomeric units to prepare Buna-s and nylon-6,6.
Buna-S
-The monomers used to prepare Buna-S polymer are 1,3-butadiene and styrene.
-The structures of 1,3-butadiene and styrene are as follows.

-The preparation of Buna-S is as follows.

-In the above reaction 1,3-butadiene reacts with styrene in presence of sodium metal and forms Buna-S.
Uses of Buna-S:
-Buna-S is widely used in coated papers.
-It is used as building applications like as a sealing and binding agent
-Moreover it is used in rubber cutting boards.
Nylon-6,6
-The monomers used to prepare Nylon-6,6 are adipic acid and hexamethylenediamine.
-The structures of adipic acid and hexamethylenediamine are as follows.

-The preparation of Nylon-6,6 is as follows.

-Nylon-6,6 is prepared by condensation polymerization of hexamethylene diamine with adipic acid at high temperature and pressure.
Uses of Nylon-6,6:
It is used in fabrication sheets, bristles for brushes.
Nylon-6,6 is waterproof in nature, so it is used to make swimwear.
Note: Buna-s is prepared through addition polymerization reactions of 1,3-butadiene and styrene. Nylon-6,6 is prepared by the condensation polymerization of adipic acid and hexamethylenediamine. In addition reaction there is no formation of side products but in condensation polymerization there is a formation of side products like methanol and water.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




