
How are absolute temperature and volume of a gas related?
Answer
561.3k+ views
Hint Charles's law gives the relation between the two parameters.
The gaseous system has the least intermolecular force of attraction and can easily alter its kinetic energy
Complete step by step solution:
So here in the question we are asked to comment on the relation between two parameters, temperature and volume.
We are very familiar with both the parameters from lower class. Now we have to relate the both parameters for a gaseous system.
So we know that the intermolecular force of attraction in gas is least and they will have high kinetic energy when they are given temperature and because of this they tend to expand the volume whereas when they are cooled or the temperature is lowered they will decrease its volume.
We could relate through real life experiments.
Take a balloon and fill it with air, so now we know that the balloon has a specific amount of gas (air) inside them and the pressure is 1 atm. Now keep the balloon in the refrigerator and take it out after a few hours and we could observe that the balloon has shrinked i.e. the volume of gas inside the balloon has decreased and if it is kept at a warmer condition, it will expand which means that the volume is expanding when the temperature has increased.
Hence we could conclude that when the temperature of a closed gaseous system is increased at constant pressure then the volume of the gaseous system is increasing and when the temperature is decreased, the volume also decreases. So the temperature and volume of the gas is directly proportional to each other. And this relation is the statement of well-known gas law which is called Charles's law.
Charles’s law defines the relation between absolute temperature and volume of a gas in a closed system at a constant pressure and the equation relating both can be written as,
$ V\alpha T$
$ V=kT$ , here k is the proportionality constant
$ \dfrac{V}{T}=k$
In the case of two cases we will write the equation as,
$ \dfrac{{{V}_{1}}}{{{T}_{1}}}=\dfrac{{{V}_{2}}}{{{T}_{2}}}$
If we plot a graph between temperature and volume we will get a straight line,
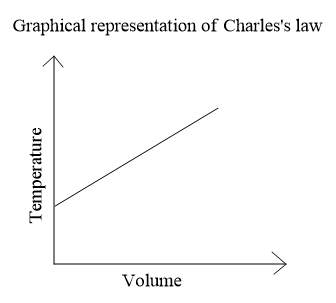
Note: We could also easily comment on the two parameters if we now the ideal gas equation which is put forward by combining the different gas laws,
$ PV=nRT$
In this equation considered the pressure is constant and the value of n be 1 and as we know R is a constant, hence we could say that the volume is directly proportional to absolute temperature.
The gaseous system has the least intermolecular force of attraction and can easily alter its kinetic energy
Complete step by step solution:
So here in the question we are asked to comment on the relation between two parameters, temperature and volume.
We are very familiar with both the parameters from lower class. Now we have to relate the both parameters for a gaseous system.
So we know that the intermolecular force of attraction in gas is least and they will have high kinetic energy when they are given temperature and because of this they tend to expand the volume whereas when they are cooled or the temperature is lowered they will decrease its volume.
We could relate through real life experiments.
Take a balloon and fill it with air, so now we know that the balloon has a specific amount of gas (air) inside them and the pressure is 1 atm. Now keep the balloon in the refrigerator and take it out after a few hours and we could observe that the balloon has shrinked i.e. the volume of gas inside the balloon has decreased and if it is kept at a warmer condition, it will expand which means that the volume is expanding when the temperature has increased.
Hence we could conclude that when the temperature of a closed gaseous system is increased at constant pressure then the volume of the gaseous system is increasing and when the temperature is decreased, the volume also decreases. So the temperature and volume of the gas is directly proportional to each other. And this relation is the statement of well-known gas law which is called Charles's law.
Charles’s law defines the relation between absolute temperature and volume of a gas in a closed system at a constant pressure and the equation relating both can be written as,
$ V\alpha T$
$ V=kT$ , here k is the proportionality constant
$ \dfrac{V}{T}=k$
In the case of two cases we will write the equation as,
$ \dfrac{{{V}_{1}}}{{{T}_{1}}}=\dfrac{{{V}_{2}}}{{{T}_{2}}}$
If we plot a graph between temperature and volume we will get a straight line,

Note: We could also easily comment on the two parameters if we now the ideal gas equation which is put forward by combining the different gas laws,
$ PV=nRT$
In this equation considered the pressure is constant and the value of n be 1 and as we know R is a constant, hence we could say that the volume is directly proportional to absolute temperature.
Recently Updated Pages
Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 Physics: Engaging Questions & Answers for Success

Master Class 11 Accountancy: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




