
$ Ar $ crystallizes in an FCC lattice with one atom at each lattice point. If the unit cell length is $ 5.311\mathop {\text{A}}\limits^ \circ $ at $ 0\;K $ , the nearest neighbour distance in $ Ar $ at $ 0\;K $ is
(A) $ 3.755\mathop {\text{A}}\limits^ \circ $
(B) $ 4.75\mathop {\text{A}}\limits^ \circ $
(C) $ 6.25\mathop {\text{A}}\limits^ \circ $
(D) $ 7.33\mathop {\text{A}}\limits^ \circ $
Answer
514.2k+ views
Hint :A smallest unit of a crystal lattice is known as a unit cell under which cubic unit cells are one of the most important unit cells. It is categorized into three types based on the arrangement of atoms in lattice i.e., simple cubic, body centred cubic and face centred cubic unit cells.
Complete Step By Step Answer:
In an FCC lattice, the atoms are placed at each corner as well as at each face centre of the unit cell. The representation of a FCC unit cell is as follows:

Where a solid sphere represents the atoms present at the corner of the unit cell while the dotted sphere represents the atoms present at the face centre of the unit cell.
As, we know for an FCC unit cell the relation between the edge length and radius of atom is as follows:
$ 4r = \sqrt 2 a $
Substituting value of edge length:
$ \Rightarrow 4r = \sqrt 2 \times 5.311\mathop {\text{A}}\limits^ \circ $
$ \Rightarrow r = 1.88\mathop {\text{A}}\limits^ \circ $
The top view of an FCC lattice will look like as follows:
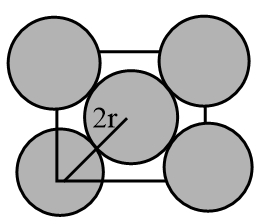
As from the figure it is clear that the nearest neighbour in an FCC lattice is at a distance of 2r, where r is the radius of the atom in an FCC unit cell.
Therefore, the nearest neighbour distance $ = 2r $
Substituting values:
$ d = 2 \times 1.88 $
$ \Rightarrow d = 3.76\mathop {\text{A}}\limits^ \circ $
Hence, option (A) is the correct answer.
Note :
It is important to note that the nearest neighbour distance in an FCC lattice can also be expressed in terms of edge length as $ d = \dfrac{a}{{\sqrt 2 }} $ . We can also find the second nearest neighbour in an FCC lattice using the expression $ d = \sqrt {\dfrac{3}{2}} a $ .
Complete Step By Step Answer:
In an FCC lattice, the atoms are placed at each corner as well as at each face centre of the unit cell. The representation of a FCC unit cell is as follows:

Where a solid sphere represents the atoms present at the corner of the unit cell while the dotted sphere represents the atoms present at the face centre of the unit cell.
As, we know for an FCC unit cell the relation between the edge length and radius of atom is as follows:
$ 4r = \sqrt 2 a $
Substituting value of edge length:
$ \Rightarrow 4r = \sqrt 2 \times 5.311\mathop {\text{A}}\limits^ \circ $
$ \Rightarrow r = 1.88\mathop {\text{A}}\limits^ \circ $
The top view of an FCC lattice will look like as follows:

As from the figure it is clear that the nearest neighbour in an FCC lattice is at a distance of 2r, where r is the radius of the atom in an FCC unit cell.
Therefore, the nearest neighbour distance $ = 2r $
Substituting values:
$ d = 2 \times 1.88 $
$ \Rightarrow d = 3.76\mathop {\text{A}}\limits^ \circ $
Hence, option (A) is the correct answer.
Note :
It is important to note that the nearest neighbour distance in an FCC lattice can also be expressed in terms of edge length as $ d = \dfrac{a}{{\sqrt 2 }} $ . We can also find the second nearest neighbour in an FCC lattice using the expression $ d = \sqrt {\dfrac{3}{2}} a $ .
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




