
Aqua-regia is a mixture of hydrochloric acid and sulphuric acid.
(A) True
(B) False
Answer
597k+ views
Hint: The mixture of nitric acid and hydrochloric acid gives a product that can dissolve gold. This product is used in cleaning gold ornaments. It is also used in cleaning glassware in labs.
Complete step by step solution:
- Aqua-regia was discovered by Jabir Bin Hayyan around the year 800AD.
- Aqua- regia is actually a corrosive acid mixture of nitric acid ($HN{{O}_{3}}$) and hydrochloric acid (HCl) in the molar ratio of 1:3.
- Aqua-regia is also known by the name- royal water. It is a yellow-orange coloured fuming liquid.
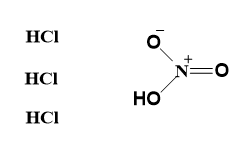
- Aqua-regia can be represented as-
- When the acids are mixed in the 3:1 ratio, the following reactions occur-
\[\begin{align}
& HN{{O}_{3}}(aq)+3HCl(aq)\to NOCl(g)+C{{l}_{2}}(g)+{{H}_{2}}O \\
& 2NOCl(g)\to 2NO(g)+C{{l}_{2}}(g) \\
& 2NO(g)+{{O}_{2}}(g)\to 2N{{O}_{2}}(g) \\
\end{align}\]
- Therefore, the answer is option (B) false. This is because aqua-regia is not a mixture of hydrochloric acid and sulphuric acid, but a mixture of hydrochloric acid and nitric acid.
Additional Information:
- Aqua-regia has a wide range of uses in both laboratories and industries.
- It is used in laboratories to clean glassware that was used for reactions containing organic compounds and metals.
- Aqua-regia is extremely explosive and has been the cause of several explosives due to mishandling.
Note: Aqua-regia is obtained only when we mix nitric acid and hydrochloric acid in the molar ratio of 1:3. Any other ratio of these acids might have similar properties, but they are not called aqua-regia.
Complete step by step solution:
- Aqua-regia was discovered by Jabir Bin Hayyan around the year 800AD.
- Aqua- regia is actually a corrosive acid mixture of nitric acid ($HN{{O}_{3}}$) and hydrochloric acid (HCl) in the molar ratio of 1:3.
- Aqua-regia is also known by the name- royal water. It is a yellow-orange coloured fuming liquid.
- Aqua-regia can be represented as-
- When the acids are mixed in the 3:1 ratio, the following reactions occur-

\[\begin{align}
& HN{{O}_{3}}(aq)+3HCl(aq)\to NOCl(g)+C{{l}_{2}}(g)+{{H}_{2}}O \\
& 2NOCl(g)\to 2NO(g)+C{{l}_{2}}(g) \\
& 2NO(g)+{{O}_{2}}(g)\to 2N{{O}_{2}}(g) \\
\end{align}\]
- Therefore, the answer is option (B) false. This is because aqua-regia is not a mixture of hydrochloric acid and sulphuric acid, but a mixture of hydrochloric acid and nitric acid.
Additional Information:
- Aqua-regia has a wide range of uses in both laboratories and industries.
- It is used in laboratories to clean glassware that was used for reactions containing organic compounds and metals.
- Aqua-regia is extremely explosive and has been the cause of several explosives due to mishandling.
Note: Aqua-regia is obtained only when we mix nitric acid and hydrochloric acid in the molar ratio of 1:3. Any other ratio of these acids might have similar properties, but they are not called aqua-regia.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




