
What is the approximate molecular weight of hemoglobin?
Answer
524.4k+ views
Hint: Molecular weight or molecular mass of any compound represents the sum of all the atoms in their respective quantities. As hemoglobin is a complex molecule, so its approximate molecular weight is taken into account. This compound contains oxygen, hydrogen, nitrogen and iron atoms.
Complete answer:
The molecular mass or molar mass of any compound consist of the sum of the masses of all the atoms present in that compound. Hemoglobin is a component of blood and is a complex of iron. It is a component of the red blood cells of blood that transports oxygen in the body. Hemoglobin is a type of metalloprotein as it contains iron (Fe) as a metal along with nitrogen.
As we know amino acids are the basic structure of proteins, these amino acids are placed in a form of alpha helices in the structure of hemoglobin. The structure of hemoglobin is a quaternary structure. The molecular formula of which is ${{C}_{2952}}{{H}_{4664}}{{O}_{832}}{{N}_{812}}{{S}_{8}}F{{e}_{4}}$ , which is calculated to be 64,458 g/mol, in which the 4 subunits constitute 16,000 g/mol each that makes up to approximately, 64,000 Daltons.
Hence, the approximate molecular weight of hemoglobin (Hb) is 64,000 Dalton.
Note:
The structure of hemoglobin consists of iron in a heterocyclic ring surrounded by 4 pyrrole molecules. Iron is present in the center to which the oxygen binds. The structure of hemoglobin is,
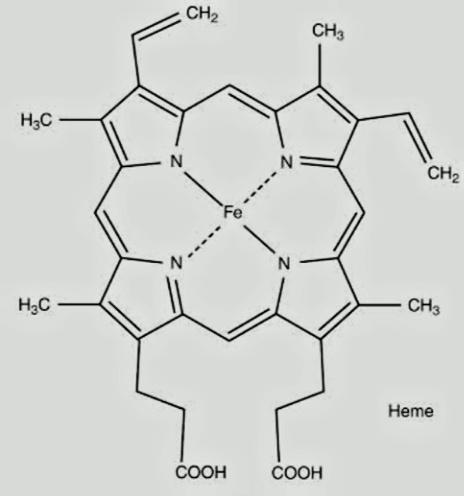
This clearly shows the complexity of the molecule. It is an important parameter for identifying the iron in the human body. Its deficiency can lead to anemia.
Complete answer:
The molecular mass or molar mass of any compound consist of the sum of the masses of all the atoms present in that compound. Hemoglobin is a component of blood and is a complex of iron. It is a component of the red blood cells of blood that transports oxygen in the body. Hemoglobin is a type of metalloprotein as it contains iron (Fe) as a metal along with nitrogen.
As we know amino acids are the basic structure of proteins, these amino acids are placed in a form of alpha helices in the structure of hemoglobin. The structure of hemoglobin is a quaternary structure. The molecular formula of which is ${{C}_{2952}}{{H}_{4664}}{{O}_{832}}{{N}_{812}}{{S}_{8}}F{{e}_{4}}$ , which is calculated to be 64,458 g/mol, in which the 4 subunits constitute 16,000 g/mol each that makes up to approximately, 64,000 Daltons.
Hence, the approximate molecular weight of hemoglobin (Hb) is 64,000 Dalton.
Note:
The structure of hemoglobin consists of iron in a heterocyclic ring surrounded by 4 pyrrole molecules. Iron is present in the center to which the oxygen binds. The structure of hemoglobin is,

This clearly shows the complexity of the molecule. It is an important parameter for identifying the iron in the human body. Its deficiency can lead to anemia.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




