
Applying the VSEPR theory, predict the shape of methyl isocyanate $CH_3NCO$. The shape of $CH_3NCO$ is predicted using valence shell electrons and Lewis dot structure.
Answer
572.1k+ views
Hint:In order to predict the geometry of molecules from a number of electron pairs surrounding their central atoms, we have a qualitative model known as Valence shell electron pair repulsion theory (VSEPR).
Complete answer:
The electron pair and lone pairs in valence shells around the central atom repel each other and tend to orient in space so as to maximize the repulsions and maximize the distance between them. Bond pairs shared by two toms and are attracted by two nuclei. Hence they occupy less space and cause less repulsion. On the other hand lone pairs are not involved in the formation of bonds, in attraction with one nucleus only therefore occupy more space and cause more repulsion Multiple bonds are treated as one single bond. The shape of molecules can be predicted from them from the number of electrons and type of electrons in the valence shell around the central atom. Double bond causes more repulsion than single bond.
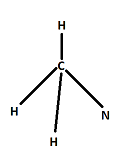
Prediction of the structure of molecules with a single central atom is easy. Here in the question we have a molecule $(CH_3NCO)$ with no single central atom. We will start from carbon attached to 3 hydrogen and 1 nitrogen. One carbon is surrounded by 4 bond pairs. So this portion of molecule should have tetrahedral geometry just like $C{H_4}$.
Now the nitrogen atom is bonded to other carbon by a double bond, therefore nitrogen is surrounded by 3 bond pairs, and then it must have one lone pair of electrons. Hence according to VSEPR theory, in this portion there should be a bent of ${120^ \circ }$, trigonal geometry.
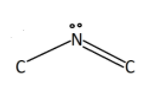
Now the next portion is carbon bonded to nitrogen and is also doubly bonded to oxygen atoms. It gives a total of 2 electron pairs to the carbon. Hence according to VSEPR theory, angle should be ${180^ \circ }$.
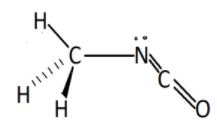
Hence the structure of methyl isocyanate will be :
Note:
Methyl isocyanate is a volatile and highly toxic molecule. In 1984, a large quantity of methyl isocyanate gas was accidently released when water leaked into storage tanks in Bhopal. The resulting highly exothermic reaction caused a rapid increase in pressure that ruptures the tanks. And they released a large amount of methyl isocyanate and killed almost 3800 people, disabled many.
Complete answer:
The electron pair and lone pairs in valence shells around the central atom repel each other and tend to orient in space so as to maximize the repulsions and maximize the distance between them. Bond pairs shared by two toms and are attracted by two nuclei. Hence they occupy less space and cause less repulsion. On the other hand lone pairs are not involved in the formation of bonds, in attraction with one nucleus only therefore occupy more space and cause more repulsion Multiple bonds are treated as one single bond. The shape of molecules can be predicted from them from the number of electrons and type of electrons in the valence shell around the central atom. Double bond causes more repulsion than single bond.
Prediction of the structure of molecules with a single central atom is easy. Here in the question we have a molecule $(CH_3NCO)$ with no single central atom. We will start from carbon attached to 3 hydrogen and 1 nitrogen. One carbon is surrounded by 4 bond pairs. So this portion of molecule should have tetrahedral geometry just like $C{H_4}$.

Now the nitrogen atom is bonded to other carbon by a double bond, therefore nitrogen is surrounded by 3 bond pairs, and then it must have one lone pair of electrons. Hence according to VSEPR theory, in this portion there should be a bent of ${120^ \circ }$, trigonal geometry.

Now the next portion is carbon bonded to nitrogen and is also doubly bonded to oxygen atoms. It gives a total of 2 electron pairs to the carbon. Hence according to VSEPR theory, angle should be ${180^ \circ }$.
Hence the structure of methyl isocyanate will be :

Note:
Methyl isocyanate is a volatile and highly toxic molecule. In 1984, a large quantity of methyl isocyanate gas was accidently released when water leaked into storage tanks in Bhopal. The resulting highly exothermic reaction caused a rapid increase in pressure that ruptures the tanks. And they released a large amount of methyl isocyanate and killed almost 3800 people, disabled many.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




