
Anthracene present in coal tar is ___________ in nature.
A) neutral
B) acidic
C) basic
D) amphoteric
Answer
585.6k+ views
Hint: Coal tar contains around \[1.5\% \] anthracene, and remains a major source of the material. Anthracene occurs naturally in coal tar and it does not react to other components of the coal tar.
Complete step by step answer:
Anthracene the polycyclic aromatic hydrocarbons are generated during combustion processes.
Let us see the structure of anthracene:
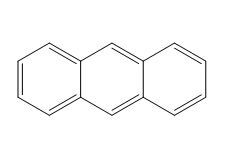
From structure, we can say that Anthracene consists of three fused benzene rings.
Hence due to the resonance stabilization energy anthracene is a highly stable compound.
We also know that Anthracene is a solid polycyclic aromatic hydrocarbon compound.
From structure, we can tell the molecular formula of Anthracene as \[{C_{14}}{H_{10}}\] .
As Anthracene is present naturally without any reaction with coal tar then it is neutral in nature.
Therefore, we can conclude that the correct answer to this question is option A.
Additional information: Exposure to humans only happens mainly through tobacco smoke and the ingestion of food contaminated with combustion products. We must know that the mineral form of anthracene is called freitalite. Anthracene can be prepared by cyclodehydration of O-methyl- or o-methylene-substituted diaryl ketones. Anthracene is biodegradable in soil and is highly responsive to degradation in the presence of sunlight. Anthracene is non-carcinogenic. For the formation of anthraquinone, the raw material is Anthracene.The variety of anthracene derivatives can have specialized uses in different fields.
Note:
Anthracene does not react with coal tar so Anthracene being acidic, basic, and amphoteric can be neglected because then the reaction with different components of coal tar and Anthracene would have taken place.
Complete step by step answer:
Anthracene the polycyclic aromatic hydrocarbons are generated during combustion processes.
Let us see the structure of anthracene:

From structure, we can say that Anthracene consists of three fused benzene rings.
Hence due to the resonance stabilization energy anthracene is a highly stable compound.
We also know that Anthracene is a solid polycyclic aromatic hydrocarbon compound.
From structure, we can tell the molecular formula of Anthracene as \[{C_{14}}{H_{10}}\] .
As Anthracene is present naturally without any reaction with coal tar then it is neutral in nature.
Therefore, we can conclude that the correct answer to this question is option A.
Additional information: Exposure to humans only happens mainly through tobacco smoke and the ingestion of food contaminated with combustion products. We must know that the mineral form of anthracene is called freitalite. Anthracene can be prepared by cyclodehydration of O-methyl- or o-methylene-substituted diaryl ketones. Anthracene is biodegradable in soil and is highly responsive to degradation in the presence of sunlight. Anthracene is non-carcinogenic. For the formation of anthraquinone, the raw material is Anthracene.The variety of anthracene derivatives can have specialized uses in different fields.
Note:
Anthracene does not react with coal tar so Anthracene being acidic, basic, and amphoteric can be neglected because then the reaction with different components of coal tar and Anthracene would have taken place.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




