
Anthracene is………….
A.Aromatic
B.Non aromatic
C.Anti-aromatic
D.Cannot be predict
Answer
580.8k+ views
Hint: Anthracene has the ability to photo dimerize with irradiation by UV light. This results in considerable changes in the physical properties of the material. According to the structure we know about the anthracene. Anthracene is solid in state and consists of polycyclic aromatic hydrocarbon. It has three fused benzene rings derived from coal tar.
Complete step by step answer:
Anthracene has two molecules in the cell. As we know anthracene is solid and mostly used in the production of red dye alizarin and other dyes. The chemical formula of anthracene is ${C_{14}}{H_{10}}$ , has a Lewis structure that is simply three benzene molecules fused together in a row. Because the electron conjugated area is shared among all carbon atoms that have a single bond as well as a double bond.
The structure of anthracene is given below:
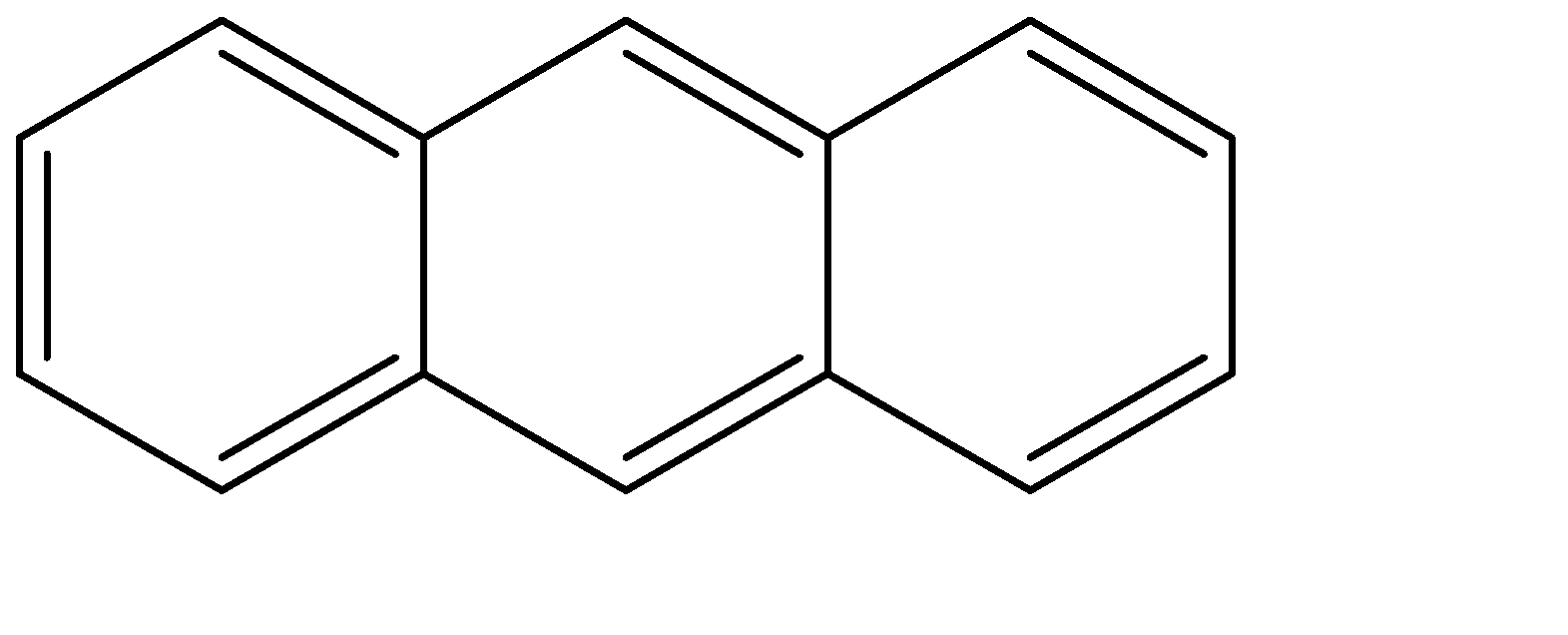
For a compound considered aromatic, it follows Huckel’s rule and overlapping p orbitals in order to be aromatic. Aromatic compounds are chemical compounds which contain conjugated planar ring systems. They are satisfied by Huckel's rule. Aromatic compounds are assemblies of benzene rings that share a common side. Aromatic compounds are generally nonpolar and immiscible with water.
Hence, option (A) is correct answer.
Note: Here we see the structure of anthracene. Benzene ring is formed in the anthracene. Molecules are cyclic, planar, completely conjugated compounds with 4n+2 electrons. Aromatic compound has a benzene ring substituted propyl group for one of the hydrogen atoms which contains aromatic.
Complete step by step answer:
Anthracene has two molecules in the cell. As we know anthracene is solid and mostly used in the production of red dye alizarin and other dyes. The chemical formula of anthracene is ${C_{14}}{H_{10}}$ , has a Lewis structure that is simply three benzene molecules fused together in a row. Because the electron conjugated area is shared among all carbon atoms that have a single bond as well as a double bond.
The structure of anthracene is given below:

For a compound considered aromatic, it follows Huckel’s rule and overlapping p orbitals in order to be aromatic. Aromatic compounds are chemical compounds which contain conjugated planar ring systems. They are satisfied by Huckel's rule. Aromatic compounds are assemblies of benzene rings that share a common side. Aromatic compounds are generally nonpolar and immiscible with water.
Hence, option (A) is correct answer.
Note: Here we see the structure of anthracene. Benzene ring is formed in the anthracene. Molecules are cyclic, planar, completely conjugated compounds with 4n+2 electrons. Aromatic compound has a benzene ring substituted propyl group for one of the hydrogen atoms which contains aromatic.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




