
Answer the following questions:
(i) Give the other two names of ethanol.
(ii) Give the structural formula of ethanol.
(iii) Give two properties of ethanol
(iv) Explain the action of phosphorus trichloride with ethanol. Write the balanced chemical equation of the above reaction.
Answer
594.9k+ views
Hint: Ethanol is a straight-chain alcohol, and its molecular formula is ${ C }_{ 2 }{ H }_{ 5 }{ OH }$. Ethanol, also called pure alcohol, grain alcohol, or drinking alcohol, is a volatile, flammable, colorless liquid.
Complete answer:
(i) The other two names of ethanol are cologne spirit and alcohol.
(ii) The structural formula of ethanol is;
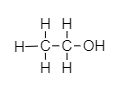
(iii) The two properties of ethanol are;
It is a monohydric primary alcohol. The melting point of ethanol is ${ -117 }^{ \circ }{ C }$ and the boiling point is ${ 78.5 }^{ \circ }{ C }$
When ethanol burns in the air it gives a blue flame, forming carbon dioxide and water. Ethanol reacts with active metals to form metal ethoxide and water.
For example: when ethanol reacts with sodium metal it will form sodium ethoxide.
(iv) The action of phosphorus trichloride with ethanol: ${ PCl }_{ 3 }$ reacts with ethanol it gives phosphorus acid and ethyl chloride. The following reaction will take place;
${ PCl }_{ 3 }{ +3C }_{ 2 }{ H }_{ 5 }{ OH\rightarrow }{ H }_{ 3 }{ PO }_{ 3 }{ +3CH }_{ 3 }{ CH }_{ 2 }{ Cl }$
Additional Information:
Uses of ethanol are:
It is used as the fluid in thermometers.
It is used in the preparation of organic compounds.
It is used in the manufacture of paints, varnishes, and medicines.
Note: The possibility to make a mistake is that when ${ PCl }_{ 3 }$ reacts with ethanol phosphorus acid will be produced while ${ PCl }_{ 5 }$ reacts with ethanol then phosphorus oxychloride will be formed. Don’t confuse between these two compounds.
Complete answer:
(i) The other two names of ethanol are cologne spirit and alcohol.
(ii) The structural formula of ethanol is;

(iii) The two properties of ethanol are;
It is a monohydric primary alcohol. The melting point of ethanol is ${ -117 }^{ \circ }{ C }$ and the boiling point is ${ 78.5 }^{ \circ }{ C }$
When ethanol burns in the air it gives a blue flame, forming carbon dioxide and water. Ethanol reacts with active metals to form metal ethoxide and water.
For example: when ethanol reacts with sodium metal it will form sodium ethoxide.
(iv) The action of phosphorus trichloride with ethanol: ${ PCl }_{ 3 }$ reacts with ethanol it gives phosphorus acid and ethyl chloride. The following reaction will take place;
${ PCl }_{ 3 }{ +3C }_{ 2 }{ H }_{ 5 }{ OH\rightarrow }{ H }_{ 3 }{ PO }_{ 3 }{ +3CH }_{ 3 }{ CH }_{ 2 }{ Cl }$
Additional Information:
Uses of ethanol are:
It is used as the fluid in thermometers.
It is used in the preparation of organic compounds.
It is used in the manufacture of paints, varnishes, and medicines.
Note: The possibility to make a mistake is that when ${ PCl }_{ 3 }$ reacts with ethanol phosphorus acid will be produced while ${ PCl }_{ 5 }$ reacts with ethanol then phosphorus oxychloride will be formed. Don’t confuse between these two compounds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




