
Answer the following questions for the below reaction:-
$Na\xrightarrow{{{H}_{2}}O}A\xrightarrow{C{{O}_{2}}}B\xrightarrow{S{{O}_{2}}}C\xrightarrow[\Delta ]{{N{{a}_{2}}S}/{{{I}_{2}}}\;}D\xrightarrow{{A{{g}^{+}}}/{salt}\;}E(complex)$
1. The compound B and C are:-
(a) $N{{a}_{2}}C{{O}_{3}},N{{a}_{2}}S{{O}_{4}}$
(b) $NaHC{{O}_{3}},N{{a}_{2}}S{{O}_{4}}$
(c) $N{{a}_{2}}C{{O}_{3}},N{{a}_{2}}S{{O}_{3}}$
(d) None of these
2. The compound D is:-
(a) $N{{a}_{2}}S{{O}_{4}}$
(b) $N{{a}_{2}}{{S}_{4}}{{O}_{6}}$
(c) $N{{a}_{2}}{{S}_{2}}{{O}_{5}}$
(d)$N{{a}_{2}}{{S}_{2}}{{O}_{3}}$
3. Oxidation no. of each ‘S’ atom in compound D:-
(a) +2, +2
(b) +4, 0
(c) +6,-2
(d) +5,-1
Answer
537.6k+ views
Hint: Attempt this question by solving the given reaction in steps. When a metal react with ${{H}_{2}}O$, it produces a base. The base on further reaction with $C{{O}_{2}}$ forms carbonate compound and ${{H}_{2}}O$. The following product forms reacts with $S{{O}_{2}}$ to give sulphide compounds. This sulphide compound when mixed with ${N{{a}_{2}}S}/{{{I}_{2}}}\;$, produces thiosulphate compound. After this calculate the oxidation state of ‘S’ atom in Thiosulphate compound by making its structure.
Complete step-by-step answer:Let’s discuss the chemical reactions given by the following compounds:-
-When sodium (metal) reacts with water, it gives Sodium Hydroxide and liberate hydrogen gas (that can be certainly checked by passing it through a beaker of soapy water which produces bubbles of ${{H}_{2}}$ gas and when burning splinter is brought near it, it makes a pop sound).
\[Na+{{H}_{2}}O\xrightarrow{{}}NaOH+{{H}_{2}}\uparrow \]
-When sodium hydroxide reacts with carbon dioxide gas, it produces sodium carbonate along with water which is shown below:-
\[2NaOH+C{{O}_{2}}\xrightarrow{{}}N{{a}_{2}}C{{O}_{3}}+{{H}_{2}}O\]
-When sulphur dioxide gas is passed through aqueous solution of sodium carbonate, displacement reaction takes place and the product formed is sodium sulphite with side products as carbon dioxide and water.
\[N{{a}_{2}}C{{O}_{3}}+{{H}_{2}}O+S{{O}_{2}}\xrightarrow{{}}N{{a}_{2}}S{{O}_{3}}+C{{O}_{2}}+{{H}_{2}}O\]
-When iodine is added to the mixture of sodium sulphite and sodium sulphide, it produces sodium thiosulphate along with sodium iodide.
\[N{{a}_{2}}S{{O}_{3}}+{N{{a}_{2}}S}/{{{I}_{2}}\xrightarrow{{}}}\;N{{a}_{2}}{{S}_{2}}{{O}_{3}}+NaI\]
-When sodium thiosulphate is mixed with silver salt, it forms the following complex of silver:-
\[2N{{a}_{2}}{{S}_{2}}{{O}_{3}}+{A{{g}^{+}}}/{salt}\;\xrightarrow{{}}N{{a}_{3}}[Ag{{({{S}_{2}}{{O}_{3}})}_{2}}]+N{{a}^{+}}salt\]
After discussing all these reactions we get to know that:-
A= $NaOH$
B= $N{{a}_{2}}C{{O}_{3}}$
C= $N{{a}_{2}}S{{O}_{3}}$
D= $N{{a}_{2}}{{S}_{2}}{{O}_{3}}$
E= \[N{{a}_{3}}[Ag{{({{S}_{2}}{{O}_{3}})}_{2}}]\]
Now let’s come to the final part i.e., oxidation state of each ‘S’ atom in compound D =$N{{a}_{2}}{{S}_{2}}{{O}_{3}}$
Oxidation state is the degree of oxidation of an atom in a compound or molecule or ion. It can be positive, negative or zero. We can calculate the oxidation state by drawing its structure as follows:-
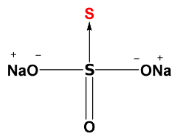
As we can see, there is a coordinate bond between 2 same atoms. Therefore the acceptor sulphur atom will have -2 oxidation state (highlighted by red color). Now, the other S atom i.e., the donor atom is surrounded by other groups as well, so we can calculate its oxidation state easily. The sum of the oxidation state of all the atoms will be equal to 0 (as the total charge on the compound is 0).
[Oxidation state of Na is +1 and O is-2]
Let’s consider x as the oxidation state of donor S atom, then:-
2(+1) + 3(-2) + x +1(-2) = 0
x = +6
So the oxidation state of acceptor S atom is -2 and donor S atom is +6.
Therefore the answers are as follows:-
1. (c) $N{{a}_{2}}C{{O}_{3}},N{{a}_{2}}S{{O}_{3}}$
2. (d) $N{{a}_{2}}{{S}_{2}}{{O}_{3}}$
3. (c) +6,-2
Note:Remember when $S{{O}_{2}}$ react with dilute $N{{a}_{2}}C{{O}_{3}}$ , it produces $NaHC{{O}_{3}}$. But this reaction takes place at ${{0}^{\circ }}C$ . So this would not be the product ‘C’. Also $NaHC{{O}_{3}}$ does not give react with ${N{{a}_{2}}S}/{{{I}_{2}}}\;$. So make the product carefully.
-While calculating oxidation state, do not directly use “x” calculation method as followed:-
2(+1) + 3(-2) + 2x=0
x = +2
This oxidation state is not the degree of oxidation of each ‘S’ atom but it is the average oxidation state of the entire ‘S’ atom in the compound.
Complete step-by-step answer:Let’s discuss the chemical reactions given by the following compounds:-
-When sodium (metal) reacts with water, it gives Sodium Hydroxide and liberate hydrogen gas (that can be certainly checked by passing it through a beaker of soapy water which produces bubbles of ${{H}_{2}}$ gas and when burning splinter is brought near it, it makes a pop sound).
\[Na+{{H}_{2}}O\xrightarrow{{}}NaOH+{{H}_{2}}\uparrow \]
-When sodium hydroxide reacts with carbon dioxide gas, it produces sodium carbonate along with water which is shown below:-
\[2NaOH+C{{O}_{2}}\xrightarrow{{}}N{{a}_{2}}C{{O}_{3}}+{{H}_{2}}O\]
-When sulphur dioxide gas is passed through aqueous solution of sodium carbonate, displacement reaction takes place and the product formed is sodium sulphite with side products as carbon dioxide and water.
\[N{{a}_{2}}C{{O}_{3}}+{{H}_{2}}O+S{{O}_{2}}\xrightarrow{{}}N{{a}_{2}}S{{O}_{3}}+C{{O}_{2}}+{{H}_{2}}O\]
-When iodine is added to the mixture of sodium sulphite and sodium sulphide, it produces sodium thiosulphate along with sodium iodide.
\[N{{a}_{2}}S{{O}_{3}}+{N{{a}_{2}}S}/{{{I}_{2}}\xrightarrow{{}}}\;N{{a}_{2}}{{S}_{2}}{{O}_{3}}+NaI\]
-When sodium thiosulphate is mixed with silver salt, it forms the following complex of silver:-
\[2N{{a}_{2}}{{S}_{2}}{{O}_{3}}+{A{{g}^{+}}}/{salt}\;\xrightarrow{{}}N{{a}_{3}}[Ag{{({{S}_{2}}{{O}_{3}})}_{2}}]+N{{a}^{+}}salt\]
After discussing all these reactions we get to know that:-
A= $NaOH$
B= $N{{a}_{2}}C{{O}_{3}}$
C= $N{{a}_{2}}S{{O}_{3}}$
D= $N{{a}_{2}}{{S}_{2}}{{O}_{3}}$
E= \[N{{a}_{3}}[Ag{{({{S}_{2}}{{O}_{3}})}_{2}}]\]
Now let’s come to the final part i.e., oxidation state of each ‘S’ atom in compound D =$N{{a}_{2}}{{S}_{2}}{{O}_{3}}$
Oxidation state is the degree of oxidation of an atom in a compound or molecule or ion. It can be positive, negative or zero. We can calculate the oxidation state by drawing its structure as follows:-

As we can see, there is a coordinate bond between 2 same atoms. Therefore the acceptor sulphur atom will have -2 oxidation state (highlighted by red color). Now, the other S atom i.e., the donor atom is surrounded by other groups as well, so we can calculate its oxidation state easily. The sum of the oxidation state of all the atoms will be equal to 0 (as the total charge on the compound is 0).
[Oxidation state of Na is +1 and O is-2]
Let’s consider x as the oxidation state of donor S atom, then:-
2(+1) + 3(-2) + x +1(-2) = 0
x = +6
So the oxidation state of acceptor S atom is -2 and donor S atom is +6.
Therefore the answers are as follows:-
1. (c) $N{{a}_{2}}C{{O}_{3}},N{{a}_{2}}S{{O}_{3}}$
2. (d) $N{{a}_{2}}{{S}_{2}}{{O}_{3}}$
3. (c) +6,-2
Note:Remember when $S{{O}_{2}}$ react with dilute $N{{a}_{2}}C{{O}_{3}}$ , it produces $NaHC{{O}_{3}}$. But this reaction takes place at ${{0}^{\circ }}C$ . So this would not be the product ‘C’. Also $NaHC{{O}_{3}}$ does not give react with ${N{{a}_{2}}S}/{{{I}_{2}}}\;$. So make the product carefully.
-While calculating oxidation state, do not directly use “x” calculation method as followed:-
2(+1) + 3(-2) + 2x=0
x = +2
This oxidation state is not the degree of oxidation of each ‘S’ atom but it is the average oxidation state of the entire ‘S’ atom in the compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




