
Answer the following questions:
(a.) Which is the strongest acid among the hydrogen halides? Give one reason. [X = F, Cl, Br, I]
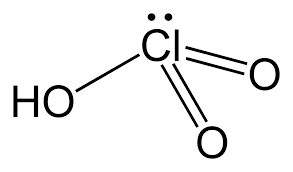
(b.) Write the structure of chloric acid (${ HClO }_{ 3 }$).
Answer
595.2k+ views
Hint: For the part (a), you just need to figure out the compound with the largest bond length and for part (b), just keep in mind it has pyramidal structure and chlorine will form a total of five bonds in this structure.
Complete step by step solution:
Let’s answer this question,
In Part (a), we have to find the strongest acid among the hydrogen halides.
The order of acidic character can be explained in terms of strength of H−X bonds, which is in the order H−I < H−Br < H−Cl < H−F. We can see that the H−I bond is weakest, therefore, HI is the strongest acid.
On the other hand, the H-F bond is strongest, hence HF is the weakest acid among all the halogen acids.
In Part (b), the structure of chloric acid (${ HClO }_{ 3 }$) is pyramidal. Chlorine makes a total of five bonds. Out of five, two are double bonds (with oxygen) and one with -OH group.
There, we answered both of these questions properly.
Note: We should know that Entropy decreases dramatically when the hydrogen fluoride reacts with water.
This thing is particularly noticeable with hydrogen fluoride because the attraction of the small fluoride ions produced imposes significant order on the surrounding water molecules and nearby hydronium ions. Also, note that this effect decreases with larger halide ions.
Complete step by step solution:
Let’s answer this question,
In Part (a), we have to find the strongest acid among the hydrogen halides.
The order of acidic character can be explained in terms of strength of H−X bonds, which is in the order H−I < H−Br < H−Cl < H−F. We can see that the H−I bond is weakest, therefore, HI is the strongest acid.
On the other hand, the H-F bond is strongest, hence HF is the weakest acid among all the halogen acids.
In Part (b), the structure of chloric acid (${ HClO }_{ 3 }$) is pyramidal. Chlorine makes a total of five bonds. Out of five, two are double bonds (with oxygen) and one with -OH group.

There, we answered both of these questions properly.
Note: We should know that Entropy decreases dramatically when the hydrogen fluoride reacts with water.
This thing is particularly noticeable with hydrogen fluoride because the attraction of the small fluoride ions produced imposes significant order on the surrounding water molecules and nearby hydronium ions. Also, note that this effect decreases with larger halide ions.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




