
Answer the following Question using the diagram below:
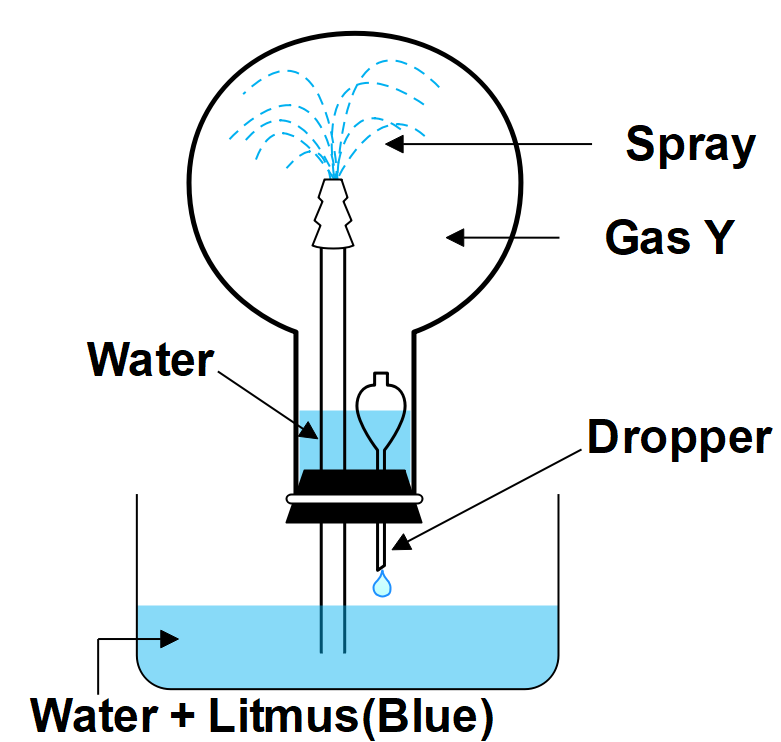
1. Name the gas.
2. What property of gas Y does this experiment show?
3. Write the equation for the laboratory preparation of this gas.
4. State its method of collection in the laboratory.
5. Explain why an aqueous solution of gas Y conducts electricity?

Answer
507k+ views
Hint: We know that the Hydrogen chloride gas is produced in the laboratory by treating sodium chloride with sulphuric acid in a reaction vessel. The hydrogen chloride produced is put into another vessel containing sulphuric acid which dries the gas.
Complete answer:
We know that pure acids in the liquid state are exceedingly poor conductors of electricity. The fact that aqueous solutions of acids conduct electricity is definite evidence of the existence of ions in these solutions. Acids undergo dissociation in aqueous solution to form $ {{H}^{+}} $ ions. When electricity is passed through an aqueous solution of an acid, the $ {{H}^{+}} $ ions reach the cathode, and each $ {{H}^{+}} $ ion picks up one electron from the cathode to form $ {{H}_{2}} $ gas.
The reaction is highly exothermic and a great amount of heat is produced. The gas produced is then absorbed into water forming pure hydrochloric acid. Thus, a low pressure is created in the flask causing the water to be pulled into the reaction flask. When added in water it ionizes, forming a proton and chloride ion. You must recall the preparation of hydrogen chloride in the laboratory
-The gas is $ HC{{l}_{(g)}}. $
-The property of the Hydrochloric acid gas is that it’s highly soluble.
-The laboratory preparation of the gas is given by; $ HCl+{{H}_{2}}O\to {{H}_{3}}O+C{{l}^{-}}. $
-The method of collection in the lab is Direct absorption of hydrogen chloride gas in water is not feasible as direct absorption leads to the back suction of water. It is caused when the rate of absorption exceeds the rate of production of the hydrogen chloride gas.
-We can conclude it as an aqueous solution of an acid that conducts electricity because, in water, an acid $ (HCl) $ dissociates to give ions. Since the current is carried out by the movement of ions, an aqueous solution of acid conducts electricity.
Note:
Remember that the most of the hydrogen chloride produced industrially is for the production of hydrochloric acid. At large scale, hydrogen chloride is produced by directly combining hydrogen gas and chlorine gas.
Complete answer:
We know that pure acids in the liquid state are exceedingly poor conductors of electricity. The fact that aqueous solutions of acids conduct electricity is definite evidence of the existence of ions in these solutions. Acids undergo dissociation in aqueous solution to form $ {{H}^{+}} $ ions. When electricity is passed through an aqueous solution of an acid, the $ {{H}^{+}} $ ions reach the cathode, and each $ {{H}^{+}} $ ion picks up one electron from the cathode to form $ {{H}_{2}} $ gas.
The reaction is highly exothermic and a great amount of heat is produced. The gas produced is then absorbed into water forming pure hydrochloric acid. Thus, a low pressure is created in the flask causing the water to be pulled into the reaction flask. When added in water it ionizes, forming a proton and chloride ion. You must recall the preparation of hydrogen chloride in the laboratory
-The gas is $ HC{{l}_{(g)}}. $
-The property of the Hydrochloric acid gas is that it’s highly soluble.
-The laboratory preparation of the gas is given by; $ HCl+{{H}_{2}}O\to {{H}_{3}}O+C{{l}^{-}}. $
-The method of collection in the lab is Direct absorption of hydrogen chloride gas in water is not feasible as direct absorption leads to the back suction of water. It is caused when the rate of absorption exceeds the rate of production of the hydrogen chloride gas.
-We can conclude it as an aqueous solution of an acid that conducts electricity because, in water, an acid $ (HCl) $ dissociates to give ions. Since the current is carried out by the movement of ions, an aqueous solution of acid conducts electricity.
Note:
Remember that the most of the hydrogen chloride produced industrially is for the production of hydrochloric acid. At large scale, hydrogen chloride is produced by directly combining hydrogen gas and chlorine gas.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




