
How anisole reacts with bromine in presence of ethanoic acid? Write the chemical equation for the reaction.
Answer
529.4k+ views
Hint: Anisole is an electron withdrawing group, it undergoes electrophilic substitution. The halogenation will take place only at the ortho and para positions for anisole and not at the meta position.
Complete Step by Step Solution: Anisole is an organic compound and we also know it by the name methoxybenzene. The chemical formula of anisole is ${{C}_{6}}{{H}_{5}}OC{{H}_{3}}$.
The methoxy group present in anisole will donate its electron density of the oxygen atom to the benzene ring and therefore the electron density on the benzene ring will increase and it will undergo electrophilic aromatic substitution, resulting in halogenation.
As we know, the methoxy group present in anisole makes it an ortho-para directing group, which means the attacking atom/group will be attached to the ortho or para positions with respect to the methoxy group in the ring.
Ethanoic acid, as we know it by the name acetic acid acts as a polar solvent for dissolving anisole.
We can write the reaction of bromination of anisole as-
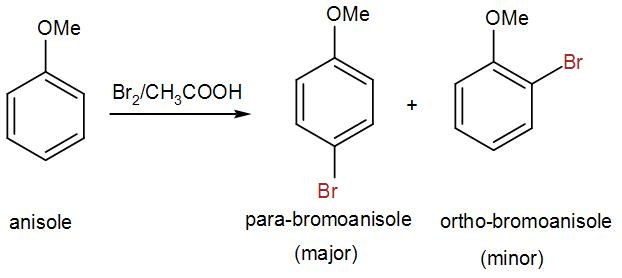
Here, we get two products, para-bromoanisole which we can also write as 1-bromo-2-methoxybenzene and the other product is ortho-bromoanisole or 1-bromo-4-methoxybenzene.
Among these two, as we can see para-bromoanisole is the major product and the yield is around 90% for it and the ortho-bromoanisole is the minor product with a very low yield. This is due to the steric hindrance in the ortho position as a bulky methoxy group is attached to the benzene ring.
Note: As we can see in the above discussion we mentioned that electron withdrawing groups increases the electron density at ortho and para position but the reason behind it can be understood from the resonance hybrid structures of anisole-
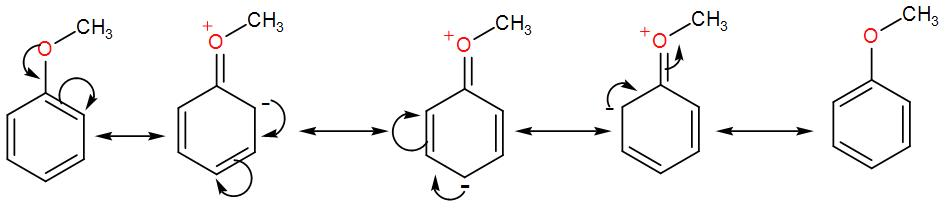
As we can see, the negative charge is passing through the ortho and the para positions therefore, these are the activated positions hence we get an ortho and a para product.
Complete Step by Step Solution: Anisole is an organic compound and we also know it by the name methoxybenzene. The chemical formula of anisole is ${{C}_{6}}{{H}_{5}}OC{{H}_{3}}$.
The methoxy group present in anisole will donate its electron density of the oxygen atom to the benzene ring and therefore the electron density on the benzene ring will increase and it will undergo electrophilic aromatic substitution, resulting in halogenation.
As we know, the methoxy group present in anisole makes it an ortho-para directing group, which means the attacking atom/group will be attached to the ortho or para positions with respect to the methoxy group in the ring.
Ethanoic acid, as we know it by the name acetic acid acts as a polar solvent for dissolving anisole.
We can write the reaction of bromination of anisole as-

Here, we get two products, para-bromoanisole which we can also write as 1-bromo-2-methoxybenzene and the other product is ortho-bromoanisole or 1-bromo-4-methoxybenzene.
Among these two, as we can see para-bromoanisole is the major product and the yield is around 90% for it and the ortho-bromoanisole is the minor product with a very low yield. This is due to the steric hindrance in the ortho position as a bulky methoxy group is attached to the benzene ring.
Note: As we can see in the above discussion we mentioned that electron withdrawing groups increases the electron density at ortho and para position but the reason behind it can be understood from the resonance hybrid structures of anisole-

As we can see, the negative charge is passing through the ortho and the para positions therefore, these are the activated positions hence we get an ortho and a para product.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Trending doubts
Draw a labelled sketch of the human eye class 12 physics CBSE

Which are the Top 10 Largest Countries of the World?

Draw ray diagrams each showing i myopic eye and ii class 12 physics CBSE

Giving reasons state the signs positive or negative class 12 physics CBSE

Explain esterification reaction with the help of a class 12 chemistry CBSE

What is defined as a solenoid Depict a diagram with class 12 physics CBSE




