
Aniline reacts with acetaldehyde to form
A. Schiff’s base
B. Carbylamine
C. Immine
D. None of these
Answer
569.4k+ views
Hint: Aniline is an example for primary amines. Primary amines react with aldehydes and ketones and form the respective products. Secondary amines cannot react with acetaldehyde and ketones. The presence of primary amines can be detected by using acetaldehyde or ketones.
Complete Solution :
- In the question it is given that which product is going to form aniline reacts with acetaldehyde (carbonyl compound).
- The structure of aniline and acetaldehyde is as follows.
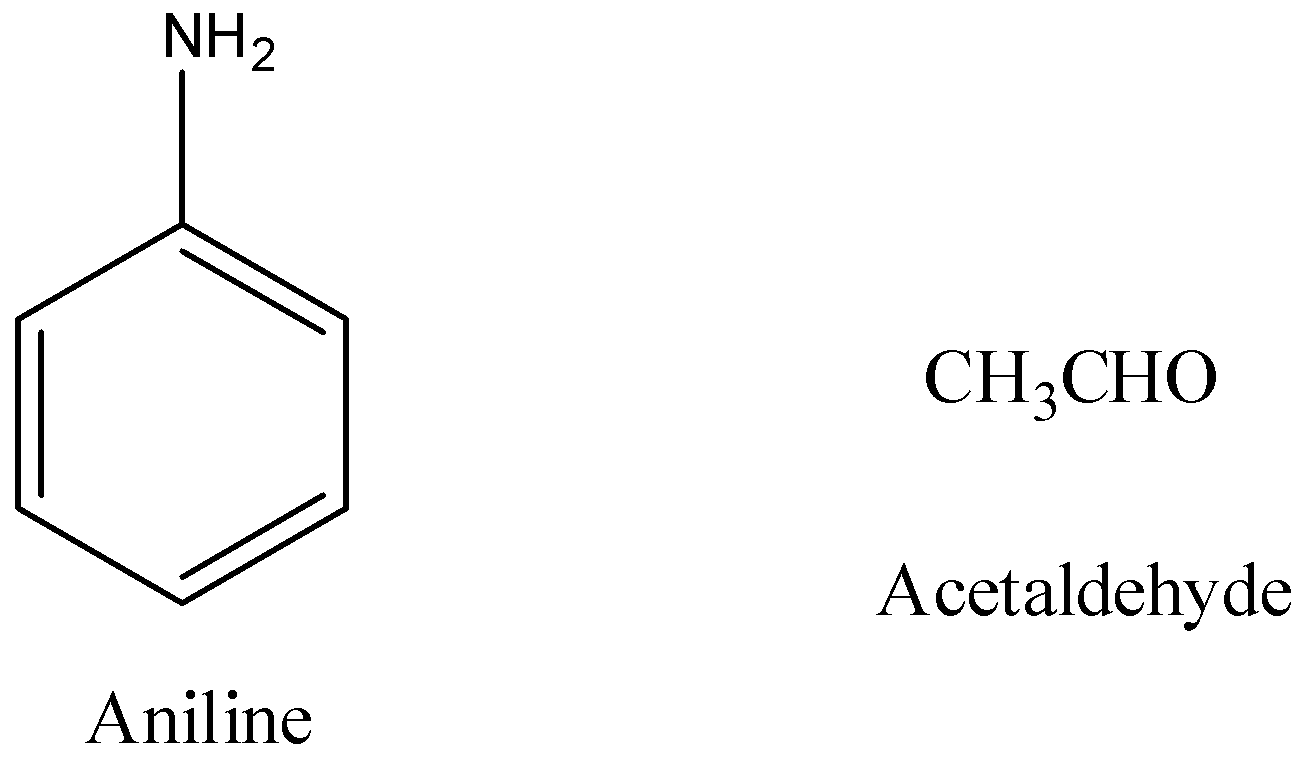
- The reaction of aniline with acetaldehyde is as follows.
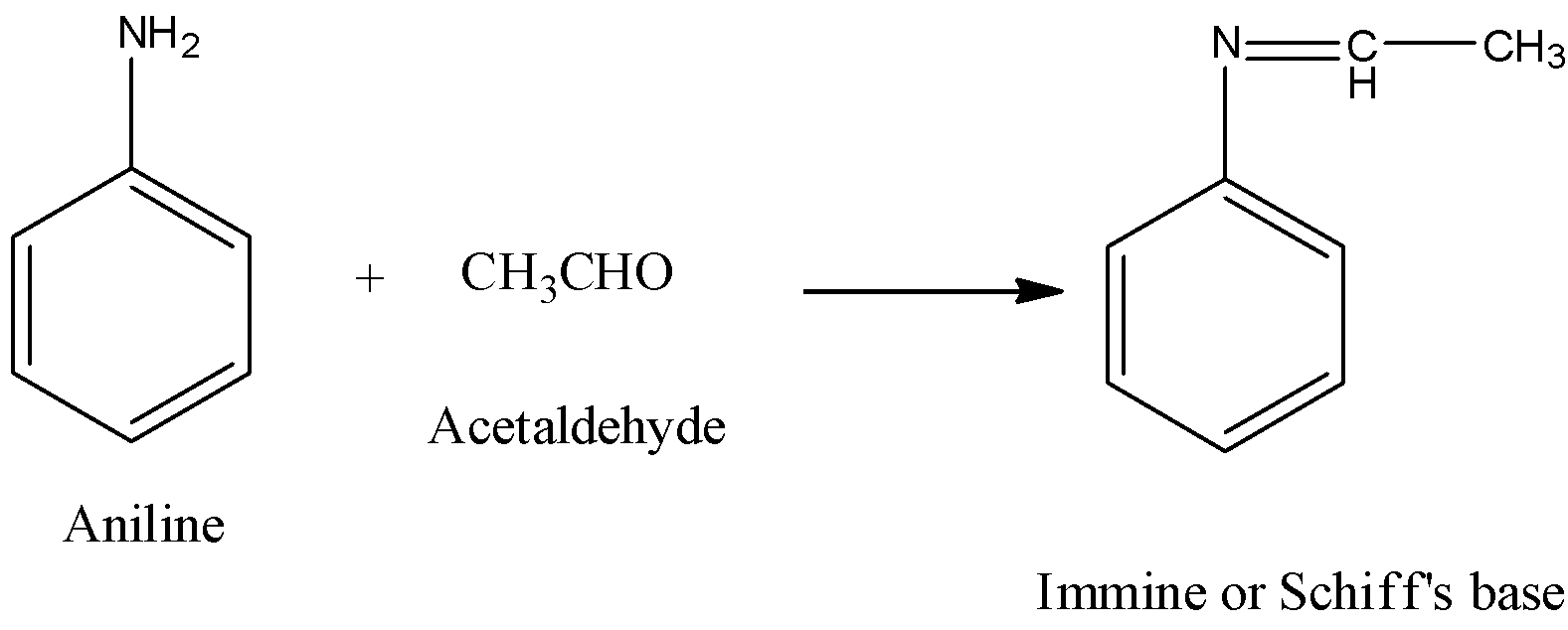
- In the above reaction aniline reacts with acetaldehyde and forms an immine compound called schiff’s base.
- Therefore the product formed when aniline reacts with acetaldehyde is schiff’s base.
So, the correct answer is “Option A”.
Additional information:
- Schiff’s base is used for antibacterial, antifungal and antimalarial purposes.
- Schiff’s base is also used as a catalyst in some organic reactions.
- Schiff’s base is used as a dye.
- Schiff’s base also acts as a corrosion inhibitor.
- Schiff’s base has wide applications in food industries also.
- It is also used for anti-inflammatory purposes.
Note: Whenever primary amines react with carbonyl compounds they produce imine groups. The carbonyl group in the molecule is going to be replaced with an imine functional group. Primary amines only form schiff’s base efficiently when compared to secondary and tertiary amines. Schiff’s bases are multipurpose compounds. Primary amine is going to condense with aldehyde or ketone to form schiff’s base.
Complete Solution :
- In the question it is given that which product is going to form aniline reacts with acetaldehyde (carbonyl compound).
- The structure of aniline and acetaldehyde is as follows.

- The reaction of aniline with acetaldehyde is as follows.

- In the above reaction aniline reacts with acetaldehyde and forms an immine compound called schiff’s base.
- Therefore the product formed when aniline reacts with acetaldehyde is schiff’s base.
So, the correct answer is “Option A”.
Additional information:
- Schiff’s base is used for antibacterial, antifungal and antimalarial purposes.
- Schiff’s base is also used as a catalyst in some organic reactions.
- Schiff’s base is used as a dye.
- Schiff’s base also acts as a corrosion inhibitor.
- Schiff’s base has wide applications in food industries also.
- It is also used for anti-inflammatory purposes.
Note: Whenever primary amines react with carbonyl compounds they produce imine groups. The carbonyl group in the molecule is going to be replaced with an imine functional group. Primary amines only form schiff’s base efficiently when compared to secondary and tertiary amines. Schiff’s bases are multipurpose compounds. Primary amine is going to condense with aldehyde or ketone to form schiff’s base.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




