
When aniline is heated with conc. ${H_2}S{O_4}$ at 455-475 $K$ ,it forms:
A. Aniline hydrogensulphate
B. Sulphanilic acid
C. Amino benzene sulphonic acid
D. Benzenesulphonic acid
Answer
572.1k+ views
Hint: benzene is a very versatile compound which shows a wide range of unique properties. Such different properties can also be seen in the benzene derivatives as well as different groups get attached to the benzene ring and thus affect the chemistry of the ring due to its electron-withdrawing or releasing nature.
Complete step by step answer:
The above reaction can be termed as the process of sulphonation. Sulphonation is the class of reactions in which the hydrogen in the arene is replaced by the sulphonic acid functional group. The reaction mechanism adopted by the reaction is the electrophilic aromatic substitution. The temperature for such reactions is generally 455-475 $K$ .When benzene is heated in the presence of fuming sulphuric acid it leads to the addition to the sulphonic group in the benzene ring and yields the product.Sulphonation is a reversible process. The reaction forms the anilinium hydrogen sulphate as the intermediate product.
The above reaction can be given as below:
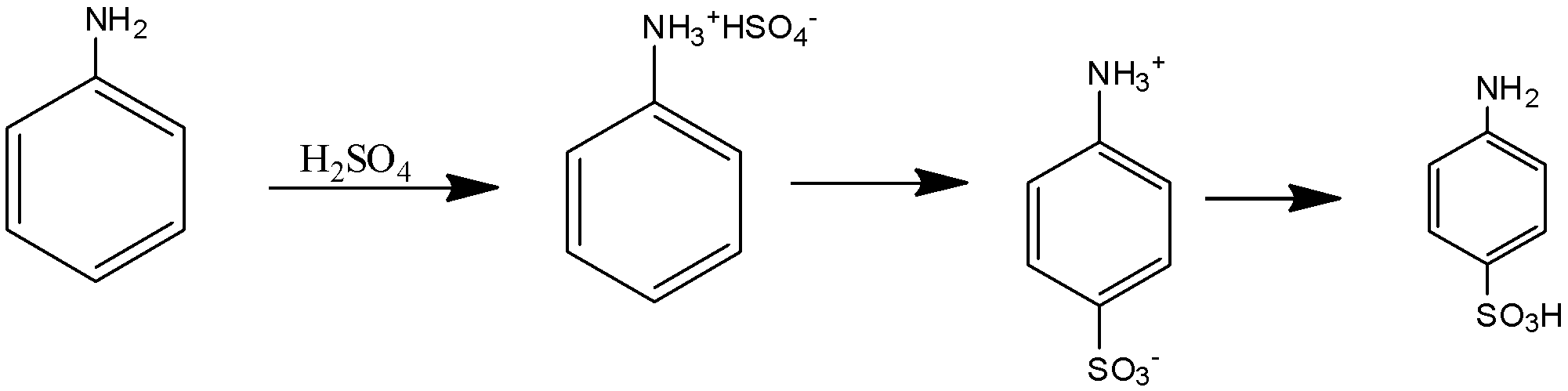
The final product is called sulphanilic acid.
So, the correct answer is Option B .
Note: The compound formed in the intermediate step is called the zwitterion also known as inner saltIt is a molecule that contains an equal number of both the positively charged and negatively charged groups. The overall charge of the zwitterion is neutral due to the presence of both the negative and positive charge in the same number.
Complete step by step answer:
The above reaction can be termed as the process of sulphonation. Sulphonation is the class of reactions in which the hydrogen in the arene is replaced by the sulphonic acid functional group. The reaction mechanism adopted by the reaction is the electrophilic aromatic substitution. The temperature for such reactions is generally 455-475 $K$ .When benzene is heated in the presence of fuming sulphuric acid it leads to the addition to the sulphonic group in the benzene ring and yields the product.Sulphonation is a reversible process. The reaction forms the anilinium hydrogen sulphate as the intermediate product.
The above reaction can be given as below:

The final product is called sulphanilic acid.
So, the correct answer is Option B .
Note: The compound formed in the intermediate step is called the zwitterion also known as inner saltIt is a molecule that contains an equal number of both the positively charged and negatively charged groups. The overall charge of the zwitterion is neutral due to the presence of both the negative and positive charge in the same number.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




