
An Unsaturated hydrocarbon is
(A)$C{{H}_{4}}$
(B)${{C}_{2}}{{H}_{5}}$
(C)${{C}_{2}}{{H}_{6}}$
(D)${{C}_{2}}{{H}_{5}}OH$
Answer
573.9k+ views
Hint: Draw the structure of the following option so that we can easily find out which one is unsaturated hydrocarbon. Unsaturated hydrocarbons are those hydrocarbons which have double or triple bonds in their structure. Hydrocarbons are nothing but compounds based on carbon and hydrogen. In saturated hydrocarbons carbon and hydrogen are only bonded by single bonds while in contrast in unsaturated hydrocarbons they are bonded by double or triple bonds also.
Complete answer:
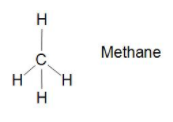
Let us see in the structure of Methane $C{{H}_{4}}$ the centre is carbon and it is bonded with four hydrogen bonds with sigma bonds (single bond).Which means it is not unsaturated hydrocarbon .
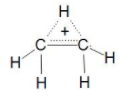
Now let us see Ethynide ${{C}_{2}}{{H}_{5}}$ here the two carbons are bonded with sigma bond and five hydrogen is bonded to them as three on one carbon and two on one carbon with sigma bond but here one carbon valency is not completed Due to which it get unstable and try to make a double bond ($\pi $-bond) so we can say that it is unsaturated hydrocarbon. Here Ethynide can be a Radical, Anion or Cation.
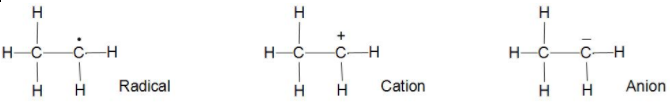
When the hydrogen goes with half of the bond or we can say takes away one electron with it then it is radical.
When the hydrogen goes away leaving the electron make it as an anion then it is an anion.
Now when the hydrogen takes both the electrons or we can say the whole bond then it is cation.
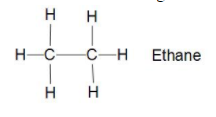
Now in the structure of Ethane ${{C}_{2}}{{H}_{6}}$ there are two carbon bonded together with sigma bond and
six hydrogen bonded three each to carbon with sigma bond (single bond). which means it is also saturated hydrocarbon with all elements bonded with sigma bonds.
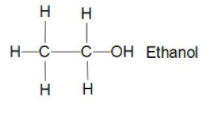
Now let us take Ethanol ${{C}_{2}}{{H}_{5}}OH$ there in its structure we can see that two carbons bonded together with sigma bond and five hydrogen bonded three to one carbon and two to another carbon with sigma bond and one hydroxyl group attached to second carbon with sigma bond only so we can say that it is also saturated hydrocarbon.
Note:
- In saturated hydrocarbons carbon and hydrogen are only bonded by single bonds while on contrary in unsaturated hydrocarbons they are bonded by double or triple bonds also.
-Here Ethynide can be radical/cation/anion. Due to which there is a formation of double bonds to protect it from being unstable.
-An atom, molecule or an ion having an unpaired valence electron is called a Radical.
Complete answer:
Let us see in the structure of Methane $C{{H}_{4}}$ the centre is carbon and it is bonded with four hydrogen bonds with sigma bonds (single bond).Which means it is not unsaturated hydrocarbon .

Now let us see Ethynide ${{C}_{2}}{{H}_{5}}$ here the two carbons are bonded with sigma bond and five hydrogen is bonded to them as three on one carbon and two on one carbon with sigma bond but here one carbon valency is not completed Due to which it get unstable and try to make a double bond ($\pi $-bond) so we can say that it is unsaturated hydrocarbon. Here Ethynide can be a Radical, Anion or Cation.

When the hydrogen goes with half of the bond or we can say takes away one electron with it then it is radical.
When the hydrogen goes away leaving the electron make it as an anion then it is an anion.
Now when the hydrogen takes both the electrons or we can say the whole bond then it is cation.

Now in the structure of Ethane ${{C}_{2}}{{H}_{6}}$ there are two carbon bonded together with sigma bond and
six hydrogen bonded three each to carbon with sigma bond (single bond). which means it is also saturated hydrocarbon with all elements bonded with sigma bonds.

Now let us take Ethanol ${{C}_{2}}{{H}_{5}}OH$ there in its structure we can see that two carbons bonded together with sigma bond and five hydrogen bonded three to one carbon and two to another carbon with sigma bond and one hydroxyl group attached to second carbon with sigma bond only so we can say that it is also saturated hydrocarbon.

Note:
- In saturated hydrocarbons carbon and hydrogen are only bonded by single bonds while on contrary in unsaturated hydrocarbons they are bonded by double or triple bonds also.
-Here Ethynide can be radical/cation/anion. Due to which there is a formation of double bonds to protect it from being unstable.
-An atom, molecule or an ion having an unpaired valence electron is called a Radical.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




